Advertisements
Advertisements
Question
Explain basic nature of amines.
Solution
Nitrogen atom of amines contains a lone pair of electrons which can be donated. Thus, amines act as Lewis bases. Amines are Lowry-Bronsted bases as they accept a proton. Thus, amines act as bases and nucleophiles.
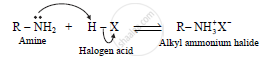
eg.
a. The reaction of ethylamine with dilute hydrochloric acid results in the formation
of ethyl ammonium chloride.
`CH_3-CH_2-NH_2+HCl⇌CH_3-CH_2NH_3^+Cl^-`
Ethylamine Ethyl ammonium
(Ethanamine) chloride
b. The reaction of aniline with dilute hydrochloric acid results in the formation of
anilinium chloride.
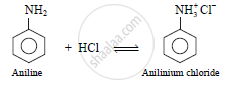
APPEARS IN
RELATED QUESTIONS
Give the structures of A, B and C in the following reactions :

Give the structures of A, B and C in the following reactions :

Write structures of compounds A and B in each of the following reactions: 
The following compound is called:
\[\begin{array}{cc}
\ce{CH3}\\
/\phantom{....}\\
\ce{CH3-N}\phantom{.............}\\
\backslash\phantom{....}\\
\phantom{.}\ce{CH3}
\end{array}\]
Which of the following is a 3° amine?
The correct IUPAC name for \[\ce{CH2 = CHCH2 NHCH3}\] is ______.
Which of the following is the weakest Brönsted base?
Give the structure of ‘A’ in the following reaction.
What is the structure and IUPAC name of the compound, allylamine?
Write down the IUPAC name of 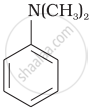
The structural formula of N-methyl aminomethane is ______.
