Advertisements
Advertisements
Question
Write a note on the self oxidation-reduction reaction of an aldehyde with a suitable example.
Solution
Cannizaro’s Reaction:-
(a) This is a characteristic reaction of those aldehydes which do not contain α -hydrogen atom.
(b) When such aldehydes are heated with concentrated alkali solutions like NaOH or KOH then simultaneous oxidation-reduction takes place.
(c) Out of two molecules of aldehyde one molecule, gets oxidised to form sodium or potassium salt of carboxylic acid and the second molecule is reduced to form corresponding primary alcohol.
(d) It is an auto oxidation-reduction reaction under the influence of base
(e) Formaldehyde and benzaldehyde undergo Cannizaro’s reaction as they do not contain α -hydrogen atom.
e.g.
\[\ce{\underset{\text{Formaldehyde(40-50%)}}{2H-CHO +NaOH}->[Redox][Reaction]\underset{\text{Sodium formate}}{HCOONa}+\underset{\text{Methanol}}{CH3}- OH}\]
\[\ce{\underset{\text{Benzaldehyde(50%)}}{2C6H5CHO +NaOH}->[Redox][Reaction]\underset{\text{Benzylalcohol}}{C6H5CH2OH} +\underset{\text{Sodiumbenzoate}}{C6H5COONa}}\]
(f) Acetaldehyde does not give this reaction since it contains α -Hydrogen atoms.
APPEARS IN
RELATED QUESTIONS
Write the chemical equation for the reaction involved in Cannizzaro reaction.
Write the chemical equations to illustrate the following name reaction:
Cannizzaro’s reaction
Describe the following:
Cannizzaro reaction
Write the reactions involved in the following reactions: Clemmensen reduction
Write the product(s) in the following reactions

Write the equations involved in the following reactions:
Etard reaction
Complete the following reactions:
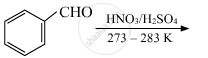
Write the chemical equations to illustrate the following name reactions:
Aldol condensation
How will you convert acetone to acetone cyanohydrin?
Write the product formed when p-nitro chlorobenzene is heated with aqueous NaOH at 443K followed by acidification?
complete the following reaction:
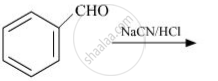
complete the following reaction:
\[\begin{array}{cc}
\phantom{...}\ce{CH3} \\
| \\
\phantom{.................}\ce{CH3-CH-COOH ->[(i) Br2/Red P4][(ii)H2O]}
\end{array}\]
Complete the following reaction:

The products obtained in the Cannizzaro reaction are
The key step in cannizzaro reaction in the inter molecular shift qf
Explain the following reaction:
Cannizzaro reaction
In the Cannizzaro reaction given below:
\[\ce{2Ph-CHO ->[OH^-] Ph-CH2OH + PhC\overset{-}{O}_2}\]
the slowest step is:
Which of the following does not give Cannizzaro reaction?
Write the chemical reaction involved in Cannizzaro reaction of methanal.
