HSC Science (General)
HSC Science (Electronics)
HSC Science (Computer Science)
Academic Year: 2016-2017
Date: July 2017
Advertisements
Which of the following is a basic oxide?
(A) SiO2
(B) P4O10
(C) MgO
(D) Al2O3
Chapter: [7.01] Group 15 Elements
In the representation of the galvanic cell, the ions in the same phase are separated by a _______.
single vertical line
comma
double vertical line
semicolon
Chapter: [0.04] Electrochemistry
An ionic crystal lattice has limiting value of radius ratio as 0.414 to 0.732; the co-ordination number of its cation is _______.
(A) 6
(B) 4
(C) 3
(D) 12
Chapter: [0.01] Solid State
The unit of rate constant for zero order reaction is _______.
(A) t–1
(B) mol dm–3 t–1
(C) mol–1 dm3 t–1
(D) mol–2 dm6 t–1
Chapter: [0.05] Chemical Kinetics [0.06] Chemical Kinetics
Calcium carbonate used in the extraction of iron acts as _______.
(A) oxidising agent
(B) reducing agent
(C) gangue
(D) flux
Chapter: [0.06] General Principles and Processes of Isolation of Elements
10.0 grams of caustic soda when dissolved in 250 cm3 of water, the resultant gram molarity of solution is _______.
(A) 0.25 M
(B) 0.5 M
(C) 1.0 M
(D) 0.1 M
Chapter: [0.04] Electrochemistry
55 L atm of work is obtained when 1.0 mole of an ideal gas is compressed isothermally from
a volume of 28.5 L to 18.5 L, the constant external pressure is
(A) 5.05 atm
(B) 5.5 atm
(C) 0.05 atm
(D) 0.55 atm
Chapter: [0.03] Chemical Thermodynamics and Energetic
State Henry’s law.
Chapter: [0.02] Solutions and Colligative Properties
What is the effect of temperature on solubility of a gas in a liquid?
Chapter: [0.02] Solutions and Colligative Properties
How is nitric acid prepared by Ostwald’s process?
Chapter: [7.01] Group 15 Elements
Classify the following solids into different types:
a. Ammonium phosphate
b. Brass
c. S8 molecule
d. Diamond
Chapter: [0.01] Solid State [0.01] Solid State
Construct a labelled diagram for the following cell:
`Zn|Zn^(2+)(1M)||H^+(1M)|H_(2(g,1atm))|Pt`
Chapter: [0.04] Electrochemistry [0.05] Electrochemistry
Explain the nature of zinc oxide with the help of the reactions.
Chapter: [7.02] Group 16 Elements
Write the names and chemical formulae of any ‘two’ minerals of aluminium.
Chapter: [0.06] General Principles and Processes of Isolation of Elements
The rate law for the reaction
`2H_(2(g))+2NO_((g))->N_(2(g))+2H_2O_((g))`
is given by rate= `K[H_2][NO]^2`
The reaction occurs in the following two steps:
a. `H_(2(g)) +2NO_((g))->N_2O_((g))+H_2O_((g))`
b. `N_2O_((g))+H_(2(g))->N_(2(g))+H_2O_((g))`
What is the role of N2O in the mechanism? What is the molecularity of each of the elementary steps?
Chapter:
Advertisements
Write mathematical equation of first law of thermodynamics for the following processes :
Adiabatic process.
Chapter: [0.03] Chemical Thermodynamics and Energetic
Write mathematical equation of first law of thermodynamics for Isochoric process.
Chapter: [0.03] Chemical Thermodynamics and Energetic
Write the mathematical expression of the First Law of Thermodynamics for Isothermal Process
Chapter: [0.04] Chemical Thermodynamics
Write the mathematical expression of the First Law of Thermodynamics for the Isobaric process.
Chapter: [0.04] Chemical Thermodynamics
From the following data for the liquid phase reaction A → B, determine the order of reaction
and calculate its rate constant
| t/s | 0 | 600 | 1200 | 1800 |
| [A]/`molL^-1` | 0.624 | 0.446 | 0.318 | 0.226 |
Chapter:
Calculate the standard enthalpy of combustion of CH3COOH(l) from the following data:
`Delta_fH^@(CO_2)=-393.3 kJ mol^-1`
`Delta_fH^@(H_2O)=-285.8 kJ mol^-1`
`Delta_fH^@(CH_3COOH)=-483.2 kJ mol^-1`
Chapter: [0.03] Chemical Thermodynamics and Energetic
Write the cell representation and calculate equilibrium constant for the following redox reaction:
`Ni_((s))+2Ag_((aq))^+ (1M)->Ni_((aq))^(2+)(1M)+2Ag_((s)) " at "25^@ C`
`E_(ni)^+`=-0.25V and `E_(Ag)^+=0.799V`
Chapter: [0.04] Electrochemistry
What is the action of concentrated sulphuric acid on phosphorous pentachloride
Chapter: [7.02] Group 16 Elements
What is the action of concentrated sulphuric acid on copper
Chapter: [7.02] Group 16 Elements
What is the action of concentrated sulphuric acid on potassium chlorate?
Chapter: [7.02] Group 16 Elements
Define Molality.
Chapter: [0.02] Solutions and Colligative Properties
Define osmotic pressure.
Chapter: [0.02] Solutions [0.02] Solutions and Colligative Properties
Write any ‘two’ advantages of calomel electrode.
Chapter: [0.04] Electrochemistry
A metal crystallises into two cubic faces namely face centered (FCC) and body centered (BCC), whose unit cell edge lengths are 3.5 Å and 3.0 Å respectively. Find the ratio of the densities of FCC and BCC.
Chapter: [0.01] Solid State
Arrange the following oxyacids of chlorine – HClO, HClO2, HClO3, and HClO4 with respect to Increasing order of thermal stability.
Chapter: [0.07] Elements of Groups 16, 17 and 18 [7.03] Group 17 Elements
Arrange the following oxyacids of chlorine – HClO, HClO2, HClO3, and HClO4 with respect to Increasing order of oxidizing power.
Chapter: [0.07] Elements of Groups 16, 17 and 18 [7.03] Group 17 Elements
An organic substance (M = 169 gram mol–1) is dissolved in 2000 cm3 of water. Its osmotic pressure at 12°C was found to be 0.54 atm. If R = 0.0821 L atm K–1 mol–1, calculate the mass of the solute.
Chapter: [0.02] Solutions and Colligative Properties
How many atoms constitute one unit cell of a face-centered cubic crystal?
Chapter: [0.01] Solid State
Distinguish between isothermal process and adiabatic process.
Chapter: [0.03] Chemical Thermodynamics and Energetic
Mention the names of various steps involved in the extraction of pure metals from their ores.
Chapter: [0.06] General Principles and Processes of Isolation of Elements
In the following
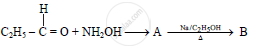
The compound ‘B’ is _______.
(A) Propan–1–amine
(B) Propan–2–amine
(C) Isopropylamine
(D) Dimethylamine
Chapter: [0.13] Amines [13.01] Amines
The stability order for carbocation is _______.
(A) 2° > 3° > 1°
(B) 3° > 2° > 1°
(C) 3° > 1° > 2°
(D) 1° > 3° > 2°
Chapter: [10.01] Haloalkanes
Effective atomic number rule is used to find _______.
(A) geometry of complex
(B) stability of complex
(C) number of isomers of complex
(D) number of possible ligands around metal ion in complex
Chapter: [0.09] Coordination Compounds [0.09] Coordination Compounds
Which one of the following ions is coloured?
Se3+
Ti4+
Zn2+
V2+
Chapter: [8.01] D-block Elements
When phenol is heated with conc. HNO3 in presence of conc. H2SO4 it yields _______.
(A) o-nitrophenol
(B) p-nitrophenol
(C) 2,4,6-trinitrophenol
(D) m-nitrophenol
Chapter: [11.02] Phenols
The secondary structure of protein is determined by _______.
(A) co-ordinate bond
(B) ionic bond
(C) hydrogen bond
(D) covalent bond
Chapter: [14.02] Proteins
Advertisements
Ethylidene dichloride when boiled with aqueous solution of NaOH yields _______.
(A) formaldehyde
(B) acetaldehyde
(C) acetone
(D) ethyl methyl ketone
Chapter: [0.11] Alcohols, Phenols and Ethers [11.01] Alcohols [11.02] Phenols [11.03] Ethers
How is phenol prepared from cumene?
Chapter: [11.02] Phenols
Write a note on the self oxidation-reduction reaction of an aldehyde with a suitable example.
Chapter: [12.01] Aldehydes and Ketones
Explain the term Antiseptics
Chapter: [16.01] Chemicals in Medicines
What happens when glucose is treated with hydroxylamine?
Chapter: [14.01] Carbohydrates
What happens when glucose is treated with hydrogen cyanide?
Chapter: [14.01] Carbohydrates
Draw structure of dichromate ion
Chapter: [8.01] D-block Elements
Draw structure of chromate ion
Chapter: [8.01] D-block Elements
Identify A and B in the following reaction:
`CH_3-Br+Mg"dry ether"/""A+CO_2"dry ether"/(H^+/(H_2O))B+Mg(Br)OH`
Chapter: [10.01] Haloalkanes [10.02] Haloarenes
How ligands are classified? Explain with suitable examples.
Chapter: [0.09] Coordination Compounds
What is lanthanoid contraction?
Chapter: [8.02] F-block Elements
Explain, why lanthanum (Z = 57) forms La3+ ion, while cerium (Z = 58) forms Ce4+ ion?
Chapter: [8.02] F-block Elements
What is the action of phenyl hydrazine on propanone?
Chapter: [0.12] Aldehydes, Ketones and Carboxylic Acids [12.01] Aldehydes and Ketones
What is the action of Zn – Hg / conc. HCl on propanone?
Chapter: [0.12] Aldehydes, Ketones and Carboxylic Acids [12.01] Aldehydes and Ketones
What is the action of Sodium bisulphite on propanone?
Chapter: [0.12] Aldehydes, Ketones and Carboxylic Acids [12.01] Aldehydes and Ketones
How is peptide linkage formed?
Chapter: [14.02] Proteins
How is nitroethane converted into ethylamine.
Chapter: [11.03] Ethers
How is nitroethane converted into N-ethylhydroxylamine
Chapter: [11.03] Ethers
How is nitroethane converted into acetic acid
Chapter: [11.03] Ethers
Write names and chemical formulae of monomers used in preparing Buna-N.
Chapter: [0.15] Polymers
How will you prepare ethanol, propan-2-ol and 2-methylpropan-2-ol from Grignard’s reagent?
Chapter: [11.01] Alcohols
Explain Optical activity
Chapter: [0.09] Coordination Compounds
Explain optical activity of lactic acid
Chapter: [0.09] Coordination Compounds
Other Solutions
Submit Question Paper
Help us maintain new question papers on Shaalaa.com, so we can continue to help studentsonly jpg, png and pdf files
Maharashtra State Board previous year question papers 12th Standard Board Exam Chemistry with solutions 2016 - 2017
Previous year Question paper for Maharashtra State Board 12th Standard Board Exam Chemistry-2017 is solved by experts. Solved question papers gives you the chance to check yourself after your mock test.
By referring the question paper Solutions for Chemistry, you can scale your preparation level and work on your weak areas. It will also help the candidates in developing the time-management skills. Practice makes perfect, and there is no better way to practice than to attempt previous year question paper solutions of Maharashtra State Board 12th Standard Board Exam.
How Maharashtra State Board 12th Standard Board Exam Question Paper solutions Help Students ?
• Question paper solutions for Chemistry will helps students to prepare for exam.
• Question paper with answer will boost students confidence in exam time and also give you an idea About the important questions and topics to be prepared for the board exam.
• For finding solution of question papers no need to refer so multiple sources like textbook or guides.
