Advertisements
Advertisements
Question
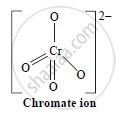
Draw structure of chromate ion
Solution
APPEARS IN
RELATED QUESTIONS
What is the geometry of chromate ion?
- Tetrahedral
- Octahedral
- Trigonal planer
- Linear
What is the molecular formula of chromyl chloride?
(A) CrO2Cl2
(B) CrOCl2
(C) CrCl3
(D) Cr2OCl2
Write observed electronic configuration of elements from first transition series having half
filled d-orbitals.
What is the action of acidified potassium dichromate on - KI
In acid medium, potassium permanganate oxidizes oxalic acid to ____________.
Which of the following statements is not true?
Permanganate ion changes to ____________ in acidic medium.
How many moles of I2 are liberated when 1 mole of potassium dichromate react with potassium iodide?
The number of moles of acidified KMnO4 required to oxidize 1 mole of ferrous oxalate (FeC2O4) is ____________.
Describe the preparation of potassium dichromate.
Complete the following.
\[\ce{C6H5CH3 ->[acidified][KMnO4]?}\]
Complete the following.
\[\ce{MnO^-_4 + Fe^2+ ->?}\]
Complete the following.
\[\ce{KMnO4 ->[\Delta][Red hot]?}\]
Complete the following.
\[\ce{Na2Cr2O7 + KCl ->?}\]
Explain why Cr2+ is strongly reducing while Mn3+ is strongly oxidizing.
Why do the d-block elements form coloured compounds?
