Advertisements
Advertisements
Question
How soap is prepared?
Solution
Soaps are formed by heating fat or oil (i.e. glyceryl esters of fatty acids) with aqueous sodium hydroxide solution. This reaction is called saponification.

During the process of hydrolysis esters of fatty acids are hydrolyzed and the soap is obtained in the colloidal form. It floats in solution as curd. It is precipited from the solution by adding sodium chloride.
APPEARS IN
RELATED QUESTIONS
Explain cationic detergents.
Write the chemical equation for preparing sodium soap from Glyceryl palmitate . Structural formulae of the compounds are given below.
(C15H31COO)3C3H5 – Glyceryl palmitate
Why do soaps not work in hard water?
Can you use soaps and synthetic detergents to check the hardness of water?
Explain the cleansing action of soaps.
Explain the mechanism of cleansing action of soaps.
Write balanced chemical equations for the action of hydrogen bromide on styrene in the presence of a peroxide
Write balanced chemical equations for the action of methyl bromide on silver propanoate
Which of the following enhances leathering property of soap?
What is a soft soap?
If soap has high alkali content it irritates skin. How can the amount of excess alkali be determined? What can be the source of excess alkali?
Why is it safer to use soap from the environmental point of view?
What is the side product of soap industry? Give reactions showing soap formation.
What is the difference between bathing soap and washing soaps?
How are transparent soaps manufactured?
What are fillers and what role these fillers play in soap?
Match the detergents given in Column I with their uses given in Column II.
| Column I | Column II |
(i) 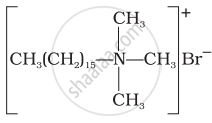 |
(a) Dishwashing powder |
(ii) 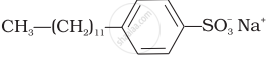 |
(b) Laundry soap |
| (iii) \[\ce{C17H33CO\overset{-}{O}\overset{+}{N}a + Na2CO3 + Rosin}\] | (c) Hair conditioners |
| (iv) \[\ce{CH3(CH2)16COO(CH2CH2O)nCH2CH2OH}\] | (d) Toothpaste |
Assertion: Transparent soaps are made by dissolving soaps in ethanol.
Reason: Ethanol makes things invisible.
Assertion: Sodium chloride is added to precipitate soap after saponification.
Reason: Hydrolysis of esters of long-chain fatty acids by alkali produces soap in colloidal form.
Green chemistry in day-to-day life is in the use of ______.
Self-cleansing windows are example of the ______.
