Advertisements
Advertisements
Question
If soap has high alkali content it irritates skin. How can the amount of excess alkali be determined? What can be the source of excess alkali?
Solution
Acid-base titration can be used to determine the excess amount of alkali in soap. The excess alkali left after hydrolysis of oils or fats can be the source of alkalinity in soap.
APPEARS IN
RELATED QUESTIONS
Following type of nom-ionic detergents are present in liquid detergents, emulsifying agents and wetting agents. Label the hydrophilic and hydrophobic parts in the molecule. Identify the functional group (s) present in the molecule.
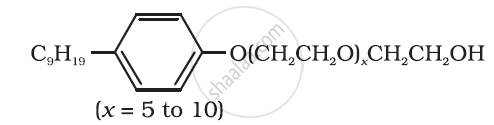
Can you use soaps and synthetic detergents to check the hardness of water?
Write balanced chemical equations for the action of methyl bromide on silver propanoate
How soap is prepared?
Which of the following enhances leathering property of soap?
What is a soft soap?
Why is it safer to use soap from the environmental point of view?
How are transparent soaps manufactured?
Match the detergents given in Column I with their uses given in Column II.
| Column I | Column II |
(i) 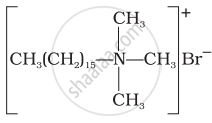 |
(a) Dishwashing powder |
(ii) 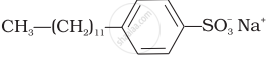 |
(b) Laundry soap |
| (iii) \[\ce{C17H33CO\overset{-}{O}\overset{+}{N}a + Na2CO3 + Rosin}\] | (c) Hair conditioners |
| (iv) \[\ce{CH3(CH2)16COO(CH2CH2O)nCH2CH2OH}\] | (d) Toothpaste |
Assertion: Transparent soaps are made by dissolving soaps in ethanol.
Reason: Ethanol makes things invisible.
