Advertisements
Advertisements
Question
What is a soft soap?
Solution
Soft soaps are potassium salts of fatty acids such as palmitic acid, stearic acid and oleic acid.
APPEARS IN
RELATED QUESTIONS
Why is bithional added to soap?
Write the chemical equation for preparing sodium soap from glyceryl oleate . Structural formulae of the compounds are given below.
(C17H32COO)3C3H5 – Glyceryl oleate
Following type of nom-ionic detergents are present in liquid detergents, emulsifying agents and wetting agents. Label the hydrophilic and hydrophobic parts in the molecule. Identify the functional group (s) present in the molecule.
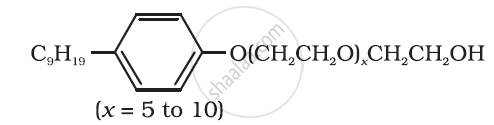
Why do soaps not work in hard water?
Explain the cleansing action of soaps.
Write balanced chemical equations for the action of methyl bromide on silver propanoate
How soap is prepared?
What is the side product of soap industry? Give reactions showing soap formation.
How are transparent soaps manufactured?
Assertion: Sodium chloride is added to precipitate soap after saponification.
Reason: Hydrolysis of esters of long-chain fatty acids by alkali produces soap in colloidal form.
