Advertisements
Advertisements
Question
What is the side product of soap industry? Give reactions showing soap formation.
Solution
Glycerol is obtained as a side product during the formation of soaps.
The process of soap formation is referred as saponification. It is the process that involves the conversion of fat or oil into soap and alcohol by action of heat in the presence of aqueous alkali.
\[\begin{array}{cc}
\ce{O}\phantom{..........................................}\\
||\phantom{..........................................}\\
\phantom{.}\ce{CH2 - O - \underset{}{C} - C17H35}\phantom{..................................}\ce{CH2 - OH}\phantom{.}\\
\phantom{..}|\phantom{..........}\ce{O}\phantom{............................................}|\phantom{..........}\\
|\phantom{..........}||\phantom{.....................................................}\\
\phantom{}\ce{CH - O - C - C17H35 + \underset{hydroxide}{\underset{Sodium}{3NaOH}} -> \underset{sterate}{\underset{Sodium}{3C17H35COONa}} + CH - OH}\phantom{.}\\
\phantom{..}|\phantom{..........}\ce{O}\phantom{............................................}|\phantom{..........}\\
|\phantom{..........}||\phantom{.....................................................}\\
\phantom{.}\ce{\underset{of stearic acid (Fat)}{\underset{Glyceryl ester}{CH2 - O - C - C17H35}}\phantom{...................................}\ce{\underset{(or Glycerine)}{\underset{Glycerol}{CH2 - OH}}}\phantom{}}
\end{array}\]
APPEARS IN
RELATED QUESTIONS
Why is bithional added to soap?
What is a soap ?
Following type of nom-ionic detergents are present in liquid detergents, emulsifying agents and wetting agents. Label the hydrophilic and hydrophobic parts in the molecule. Identify the functional group (s) present in the molecule.
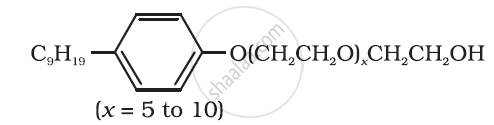
Can you use soaps and synthetic detergents to check the hardness of water?
Write balanced chemical equations for the action of hydrogen bromide on styrene in the presence of a peroxide
Write balanced chemical equations for the action of methyl bromide on silver propanoate
Which of the following enhances leathering property of soap?
If soap has high alkali content it irritates skin. How can the amount of excess alkali be determined? What can be the source of excess alkali?
Match the soaps given in Column I with items given in Column II.
| Column I | Column II |
| (i) Soap chips | (a) dried miniature soap bubbles |
| (ii) Soap granules | (b) small broken pieces of soap formed from melted soaps |
| (iii) Soap powder | (c) soap powder + abrasives + builders \[\ce{(Na2CO3,Na3PO4)}\] |
| (iv) Scouring soap | (d) soap powder + builders like \[\ce{Na2CO3}\] and \[\ce{Na3PO4}\] |
Assertion: Transparent soaps are made by dissolving soaps in ethanol.
Reason: Ethanol makes things invisible.
