Advertisements
Advertisements
Questions
Explain the mechanism of cleansing action of soaps.
Explain with diagram the mechanism of the cleansing action of soaps.
Solution 1
Mechanism of cleansing actum of soaps
Soap and detergents have two parts, a long chain, of hydrocarbon tail soluble in oil and other part (head) soluble in water
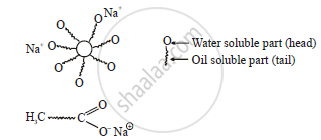
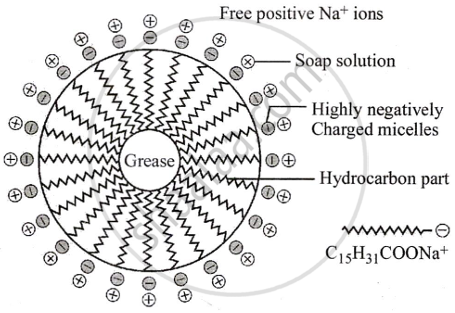
- When soap is added to an oily part of cloth as a vessel, the hydrocarbon part of the soap dissolves in oil and the ionic end of the soap dissolves in water.
- The soap molecules form micelles where one of the molecules is towards the oil droplet, while the ionic end faces outside.
- This results in an emulsion in water.
- The soap micelles assist in dissolving the dirt in water. Thus we can wash our clothes.
Solution 2
RELATED QUESTIONS
Why is bithional added to soap?
What is a soap ?
Write the chemical equation for preparing sodium soap from glyceryl oleate . Structural formulae of the compounds are given below.
(C17H32COO)3C3H5 – Glyceryl oleate
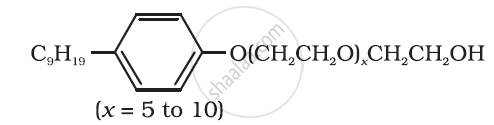
Following type of nom-ionic detergents are present in liquid detergents, emulsifying agents and wetting agents. Label the hydrophilic and hydrophobic parts in the molecule. Identify the functional group (s) present in the molecule.
Why do soaps not work in hard water?
Explain the cleansing action of soaps.
Write balanced chemical equations for the action of methyl bromide on silver propanoate
How soap is prepared?
Which of the following enhances leathering property of soap?
Glycerol is added to soap. It functions ______.
What is the difference between bathing soap and washing soaps?
How are transparent soaps manufactured?
Match the soaps given in Column I with items given in Column II.
| Column I | Column II |
| (i) Soap chips | (a) dried miniature soap bubbles |
| (ii) Soap granules | (b) small broken pieces of soap formed from melted soaps |
| (iii) Soap powder | (c) soap powder + abrasives + builders \[\ce{(Na2CO3,Na3PO4)}\] |
| (iv) Scouring soap | (d) soap powder + builders like \[\ce{Na2CO3}\] and \[\ce{Na3PO4}\] |
Match the detergents given in Column I with their uses given in Column II.
| Column I | Column II |
(i)  |
(a) Dishwashing powder |
(ii) 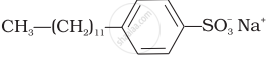 |
(b) Laundry soap |
| (iii) \[\ce{C17H33CO\overset{-}{O}\overset{+}{N}a + Na2CO3 + Rosin}\] | (c) Hair conditioners |
| (iv) \[\ce{CH3(CH2)16COO(CH2CH2O)nCH2CH2OH}\] | (d) Toothpaste |
Assertion: Transparent soaps are made by dissolving soaps in ethanol.
Reason: Ethanol makes things invisible.
Assertion: Sodium chloride is added to precipitate soap after saponification.
Reason: Hydrolysis of esters of long-chain fatty acids by alkali produces soap in colloidal form.
Which of the following is not a correct statement?
Green chemistry in day-to-day life is in the use of ______.
Self-cleansing windows are example of the ______.
