English Medium
Academic Year: 2023-2024
Date & Time: 2nd March 2024, 10:30 am
Duration: 3h
Advertisements
General Instructions:
- This question paper contains 39 questions, All questions are compulsory.
- This question paper is divided into five Sections - Section A, B, C, D and E.
- Section A: Question Nos. 1 to 20 are multiple choice questions. Each question carries 1 mark.
- Section B: Question Nos. 21 and 26 are very short answer type questions. Each question carries 2 marks. Answer to these questions should be in the range of 30 to 50 words.
- Section C: Question Nos. 27 to 33 are short answer type questions. Each question carries 3 marks. Answer to these questions should be in the range of 50 to 80 words.
- Section D: Question Nos. 34 to 36 are long answer type questions. Each question carries 5 marks. Answer to these questions should be in the range of 80 to 120 words.
- Section E: Question Nos. 37 to 39 are 3 source-based/case-based units of assessment carrying 4 marks each with sub-parts.
- There is no overall choice. However, an internal choice has been provided in some sections. Only one of the alternatives has to be attempted in such questions.
Select from the following a decomposition reaction in which source of energy for decomposition is light:
\[\ce{2FeSO4 -> Fe2O3 + SO2 + SO3}\]
\[\ce{2H2O -> 2H2 + O2}\]
\[\ce{2AgBr -> 2Ag + Br2}\]
\[\ce{CaCO3 -> CaO + CO2}\]
Chapter:
Oxides of aluminium and zinc are ______.
acidic
basic
amphoteric
neutral
Chapter:
Consider the following compounds:
\[\ce{FeSO4 ; CuSO4 ; CaSO4; Na2CO3}\]
The compound having maximum number of water of crystallisation in its crystalline form in one molecule is ______.
\[\ce{FeSO4}\]
\[\ce{CuSO4}\]
\[\ce{CaSO4}\]
\[\ce{Na2CO3}\]
Chapter:
The name and formula of third member of homologous series of alkyne is ______.
Propyne \[\ce{C3H6}\]
Propyne \[\ce{C3H4}\]
Butyne \[\ce{C4H8}\]
Butyne \[\ce{C4H6}\]
Chapter:
A metal and a non-metal that exists in liquid state at the room temperature are respectively:
Bromine and Mercury
Mercury and Iodine
Mercury and Bromine
Iodine and Mercury
Chapter:
\[\ce{MnO2 + 4HCl -> MnCl2 + 2H2O + Cl2}\]
The reaction given above is a redox reaction because in this case ______.
\[\ce{MnO2}\] is oxidised and \[\ce{HCl}\] is reduced.
\[\ce{HCl}\] is oxidised.
\[\ce{MnO2}\] is reduced.
\[\ce{MnO2}\] is reduced and \[\ce{HCl}\] is oxidised.
Chapter:
When 2 mL of sodium hydroxide solution is added to few pieces of granulated zinc in a test tube and then warmed, the reaction that occurs can be written in the form of a balanced chemical equation as:
\[\ce{NaOH + Zn -> NaZnO2 + H2O}\]
\[\ce{2NaOH + Zn -> Na2ZnO2 + H2}\]
\[\ce{2NaOH + Zn -> NaZnO2 + H2}\]
\[\ce{2NaOH + Zn -> Na2ZnO2 + H2O}\]
Chapter:
Which one of the following statements is NOT true?
DNA carries the information for inheritance of features from parents to the next generation.
DNA is the information source for making proteins.
Change in the information leads to different proteins.
Features will remain the same even if the protein changes.
Chapter:
In a nerve cell, the site where the electrical impulse is converted into a chemical signal is known as ______.
Axon
Dendrites
Neuromuscular junction
Cell body
Chapter:
Chromosomes:
- carry hereditary information from parents to the next generation.
- are thread like structures located inside the nucleus of an animal cell.
- always exist in pairs in human reproductive cells.
- are involved in the process of cell division.
The correct statements are:
i and ii
iii and iv
i, ii and iv
i and iv
Chapter:
A stomata closes when:
- it needs carbon dioxide for photosynthesis.
- it does not need carbon dioxide for photosynthesis.
- water flows out of the guard cells.
- water flows into the guard cells.
The correct reason(s) in this process is/are ______.
i only
i and iii
ii and iii
ii and iv
Chapter:
In which of the following organisms, multiple fission is a means of asexual reproduction?
Yeast
Leishmania
Paramoecium
Plasmodium
Chapter:
At what distance from a convex lens should an object be placed to get an image of the same size as that of the object on a screen?
Beyond twice the focal length of the lens.
At the principal focus of the lens.
At twice the focal length of the lens.
Between the optical centre of the lens and its principal focus.
Chapter:
The lens system of the human eye forms an image on a light sensitive screen, which is called as ______.
Cornea
Ciliary muscles
Optic nerves
Retina
Chapter:
The pattern of the magnetic field produced inside a current carrying solenoid is:


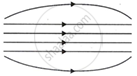

Chapter:
Identify the food chain in which the organisms of the second trophic level are missing.
Grass, goat, lion
Zooplankton, phytoplankton, small fish, large fish
Tiger, grass, snake, frog
Grasshopper, grass, snake, frog, eagle
Chapter:
Assertion (A): The rate of breathing in aquatic organisms is much faster than in terrestrial organisms.
Reason (R): The amount of oxygen dissolved in water is very high as compared to the amount of oxygen in air.
Both Assertion (A) and Reason (R) are true and Reason (R) is the correct explanation of the Assertion (A).
Both Assertion (A) and Reason (R) are true, but Reason (R) is not the correct explanation of the Assertion (A).
Assertion (A) is true, but Reason (R) is false.
Assertion (A) is false, but Reason (R) is true.
Chapter:
Assertion (A): The rainbow is a natural spectrum of sunlight in the sky.
Reason (R): Rainbow is formed in the sky when the sun is overhead and water droplets are also present in air.
Both Assertion (A) and Reason (R) are true and Reason (R) is the correct explanation of the Assertion (A).
Both Assertion (A) and Reason (R) are true, but Reason (R) is not the correct explanation of the Assertion (A).
Assertion (A) is true, but Reason (R) is false.
Assertion (A) is false, but Reason (R) is true.
Chapter:
Assertion (A): Accumulation of harmful chemicals is maximum in the organisms at the highest trophic level of a food chain.
Reason (R): Harmful chemicals are sprayed on the crops to protect them from diseases and pests.
Both Assertion (A) and Reason (R) are true and Reason (R) is the correct explanation of the Assertion (A).
Both Assertion (A) and Reason (R) are true, but Reason (R) is not the correct explanation of the Assertion (A).
Assertion (A) is true, but Reason (R) is false.
Assertion (A) is false, but Reason (R) is true.
Chapter:
Assertion (A): Hydrogen gas is not evolved when zinc reacts with nitric acid.
Reason (R): Nitric acid oxidises the hydrogen gas produced to water and itself gets reduced.
Both Assertion (A) and Reason (R) are true and Reason (R) is the correct explanation of the Assertion (A).
Both Assertion (A) and Reason (R) are true, but Reason (R) is not the correct explanation of the Assertion (A).
Assertion (A) is true, but Reason (R) is false.
Assertion (A) is false, but Reason (R) is true.
Chapter:
Why don’t two magnetic lines of force intersect each other?
Chapter: [0.12] Magnetic Effects of Electric Current
How is a uniform magnetic field in a given region represented ? Draw a diagram in support of your answer.
Chapter:
Show how you would connect three resistors each of resistance 6 Ω, so that the combination has a resistance of 9 Ω. Also justify your answer.
Chapter:
In the given circuit calculate the power consumed in watts in the resistor of 2 Ω : 2
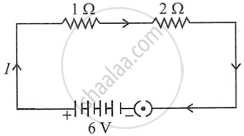
Chapter:
A ray of light falls making an angle of incidence 0 on the surface of a glass slab. Draw a labelled ray diagram to show its path. Also mark lateral displacement on it.
Chapter:
Advertisements
In which region of the brain is medulla located?
Chapter:
Write the function of Medulla obiongata.
Chapter: [0.06] Control and Co-ordination [0.06] Control and Co-ordination
In which region of the brain is cerebrum located?
Chapter:
Name the function of cerebrum.
Chapter: [0.06] Control and Co-ordination
Name a hormone that promotes the growth of tendrils and explain how they help a pea plant to climb up other plants.
Chapter:
Mention the pathway of urine in our body starting from the organ of its formation to its excretion.
Chapter:
What will happen if the tubular part of the nephron does not work properly?
Chapter:
Translate the following statement into a chemical equation and then balance it:
Solution of barium chloride and aluminium sulphate in water react to give insoluble barium sulphate and the solution of aluminium chloride.
Chapter:
Translate the following statement into a chemical equation and then balance it:
Aluminium metal reacts with steam to give aluminium oxide and hydrogen gas.
Chapter:
The pH of a sample of tomato juice is 4.6. How is this juice likely to be in taste? Give reason to justify your answer.
Chapter:
How do we differentiate between a strong acid and a weak base in terms of ion-formation in aqueous solutions?
Chapter:
The acid rain can make the survival of aquatic animals difficult. How?
Chapter:
Write one chemical equation for the chemical reaction in which the following has taken place:
Change in colour
Mention colour change along with equation.
Chapter:
Write one chemical equation for the chemical reaction in which the following has taken place:
Change in temperature
Mention temperature change (rise/fall) along with equation.
Chapter:
Write one chemical equation for the chemical reaction in which the following has taken place:
Formation of precipitate
Mention compound precipitated along with equation.
Chapter:
Define the following:
Reflex action
Chapter: [0.06] Control and Co-ordination
With the help of a flow chart, show the path of a reflex action such as sneezing.
Chapter:
In the context of the statement "chlorophyll is necessary for photosynthesis" answer the following questions:
- What are variegated leaves? Give an example.
- When leaf is boiled in alcohol, what happens to the colour of the leaf and the colour of the solution?
- In what form is the carbohydrate produced, stored in the plant ? Why is chlorophyll necessary for photosynthesis?
Chapter:
Plants → Deer → Lion
In the given food chain, what will be the impact of removing all the organisms of second trophic level on the first and third trophic level? Will the impact be the same for the organisms of the third trophic level in the above food chain if they were present in a food web? Justify.
Chapter:
A gas 'X' which is a deadly poison is found at the higher levels of atmosphere and performs an essential function.
Name the gas and write the function performed by this gas in the atmosphere. Which chemical is linked to the decrease in the level of this gas? What measures have been taken by an international organisation to check the depletion of the layer containing this gas?
Chapter:
A narrow beam, PQ of white light is passing through a glass prism ABC as shown in the diagram.
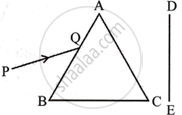 |
Draw a ray diagram to show the emergent beam as it falls on the screen DE. Also write the phenomenon involved and its cause. Using the second law of refraction state which colour of light must have the highest value of refractive index amongst seven visible colours of light. Justify your answer.
Chapter:
Name safety measures commonly used in electric circuits and appliances.
Chapter: [0.12] Magnetic Effects of Electric Current
The power rating of an electric oven is 220 V; 2 kW. If it is used in a domestic electric circuit of current rating of 5A, what result do you expect? Justify your answer with necessary calculations.
Chapter:
Advertisements
Identify the functional groups present in the following carbon compounds:
| \[\begin{array}{cc} \phantom{..}\ce{H}\phantom{...}\ce{O}\phantom{...}\ce{H}\phantom{..}\\ |\phantom{....}||\phantom{....}|\\ \ce{H - C - C - C - H}\\ |\phantom{.........}|\\ \ce{H}\phantom{........}\ce{H}\\ \end{array}\] |
\[\begin{array}{cc} \phantom{..}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{OH}\phantom{}\\ \phantom{}|\phantom{....}|\phantom{....}|\phantom{}\\ \ce{H - C - C - C = O}\\ \phantom{.}|\phantom{....}|\phantom{......}\\ \ce{H}\phantom{...}\ce{H}\phantom{.....}\\ \end{array}\] |
| (I) | (II) |
Chapter:
What happens when ethanol reacts with acidified potassium dichromate solution? Write chemical equation for the reaction. Why is this reaction considered an oxidation reaction?
Chapter:
What happens when ethanoic acid reacts with sodium hydroxide? Write equation of the reaction involved.
Chapter: [0.04] Carbon and its Compounds
Describe method of preparation of soap giving chemical equation for the reaction involved.
Chapter:
Explain the mechanism of cleansing action of soaps.
Chapter:
Express electric power in terms of potential difference (V) and resistance (R).
Chapter:
An electric oven is designed to work on the mains voltage of 220 V. This oven consumes 11 units of electrical energy in 5 hours. Calculate:
- power rating of the oven
- current drawn by the oven
- resistance of the oven when it is red hot
Chapter:
Write the relation between resistance R and electrical resistivity (ρ) of the material of a conductor in the shape of cylinder of length I and area of cross-section (a). Hence, derive the SI unit of electrical resistivity.
Chapter:
The resistance of a metal wire of length 3 m is 60 Ω. If the area of cross-section of the wire is 4 x 10−2 m2, calculate the electrical resistivity of the wire.
Chapter:
State how would electrical resistivity be affected if the wire (of part 'ii') is stretched so that its length is doubled. Justify your answer.
Chapter:
Name three techniques/devices used by human females to avoid pregnancy. Mention the side effects caused by each.
Chapter:
What will happen if in a human female fertilisation takes place?
Chapter:
What will happen if in a human female an egg is not fertilised?
Chapter:
Draw a diagram showing spore formation in Rhizopus and label the (a) reproductive and (b) non-reproductive parts. Why does Rhizopus not multiply on a dry slice of bread?
Chapter:
Name and explain the process by which reproduction takes place in Hydra.
Chapter:
Study the data given below showing the focal length of three concave mirrors A, B and C and the respective distances of objects placed in front of the mirrors:
| Case | Mirror | Focal Length (cm) |
Object Distance (cm) |
| 1 | A | 20 | 45 |
| 2 | B | 15 | 30 |
| 3 | C | 30 | 20 |
- In which one of the above cases the mirror will form a diminished image of the object? Justify your answer. 1
- List two properties of the image formed in case 2. 1
-
- What is the nature and size of the image formed by mirror C ? Draw ray diagram to justify your answer. 2
OR - An object is placed at a distance of 18 cm from the pole of a concave mirror of focal length 12 cm. Find the position of the image formed in this case. 2
- What is the nature and size of the image formed by mirror C ? Draw ray diagram to justify your answer. 2
Chapter:
| Mendel worked out the rules of heredity by working on garden pea using a number of visible contrasting characters. He conducted several experiments by making a cross with one or two pairs of contrasting characters of pea plant. On the basis of his observations, he gave some interpretations which helped to study the mechanism of inheritance. |
- When Mendel crossed pea plants with pure tall and pure short characteristics to produce F1 progeny, which two observations were made by him in F1 plants? 1
- Write one difference between dominant and recessive trail. 1
-
- In a cross with two pairs of contrasting characters
Mendel observed 4 types of combinations in F2 generation. By which method did he obtain F2 generation? Write the ratio of the parental combinations obtained and what conclusions were drawn from this experiment? 2RRYY × rryy (Round Yellow) (Wrinkled Green)
OR - Justify the statement:
"It is possible that a trait is inherited but may not be expressed." 2
- In a cross with two pairs of contrasting characters
Chapter:
| The metals produced by various reduction processes are not very pure. They contain impurities, which must be removed to obtain pure metals. The most widely used method for refining impure metals is electrolytic refining. |
- What is the cathode and anode made of in the refining of copper by this process? (1)
- Name the solution used in the above process and write its formula. (1)
-
- How copper gets refined when electric current is passed in the electrolytic cell? (2)
OR - You have two beakers 'A' and 'B' containing copper sulphate solution. What would you observe after about 2 hours if you dip a strip of zinc in beaker 'X and a strip of silver in beaker 'B'? Give reason for your observations in each case. (2)
- How copper gets refined when electric current is passed in the electrolytic cell? (2)
Chapter:
Other Solutions
Submit Question Paper
Help us maintain new question papers on Shaalaa.com, so we can continue to help studentsonly jpg, png and pdf files
CBSE previous year question papers Class 10 Science with solutions 2023 - 2024
Previous year Question paper for CBSE Class 10 Science-2024 is solved by experts. Solved question papers gives you the chance to check yourself after your mock test.
By referring the question paper Solutions for Science, you can scale your preparation level and work on your weak areas. It will also help the candidates in developing the time-management skills. Practice makes perfect, and there is no better way to practice than to attempt previous year question paper solutions of CBSE Class 10.
How CBSE Class 10 Question Paper solutions Help Students ?
• Question paper solutions for Science will helps students to prepare for exam.
• Question paper with answer will boost students confidence in exam time and also give you an idea About the important questions and topics to be prepared for the board exam.
• For finding solution of question papers no need to refer so multiple sources like textbook or guides.
