Advertisements
Advertisements
Question
What happens when glucose is treated with hydroxylamine?
Solution
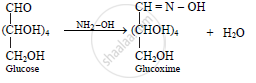
Action of hydroxylamine (NH2OH) on glucose: The reaction of glucose with
hydroxylamine gives an oxime. This indicates the presence of carbonyl group.
APPEARS IN
RELATED QUESTIONS
Write the reaction that indicates the presence of -CHO group in glucose
Draw the simple Fisher projection formulae of D - (+) - glucose and D - (-) - fructose
How many moles of acetic anhydride will be required to form glucose pentaacetate from 2M of glucose?
(a) 2
(b) 5
(c) 10
(d) 2.5
Enumerate the reactions of D-glucose which cannot be explained by its open chain structure.
Maltose is a
(a) Polysaccharide
(b) Disaccharide
(c) Trisaccharide
(d) Monosaccharide
Enlist the properties of glucose that can not be explained on the basis of open chain structure of it
The spatial arrangement of the given molecule is denoted by:

The number of asymmetric carbon atom(s) below the figure is/are

Acetylation of glucose yields ____________.
Glucose does not give Schiff’s test because of the formation of cyclic ____________.
Which of the following statements is incorrect regarding glucose?
Glucose does not react with ____________.
Glucose is found to exist in two different α and β crystalline forms. These forms can be obtained by:
(i) The α form of glucose is obtained by crystallisation from a concentrated solution of glucose at 303 K.
(ii) The β form of glucose is obtained by crystallisation from a concentrated solution of glucose at 303 K.
(iii) The β form is obtained by crystallisation from hot and saturated aqueous solution at 371 K.
(iv) The α form is obtained by crystallisation from hot and saturated aqueous solution at 371 K.
Reduction of glucose by HI suggest that ____________.
The α-D glucose and β-D glucose differ from each other due to difference in carbon atom with respect to its ____________.
The letter D and L in carbohydrates represent ____________.
Which of the following pairs represents anomers?
Three structures are given below in which two glucose units are linked. Which of these linkages between glucose units are between C1 and C4 and which linkages are between C1 and C6?
| (I) |  |
| (II) | 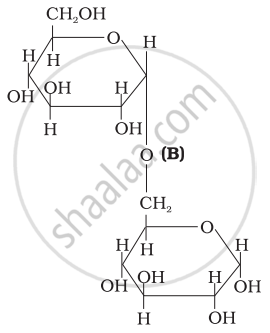 |
| (III) | 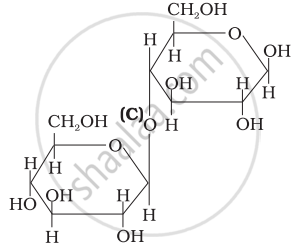 |
How will you distinguish 1° and 2° hydroxyl groups present in glucose? Explain with reactions.
Assertion: D (+) – Glucose is dextrorotatory in nature.
Reason: ‘D’ represents its dextrorotatory nature.
On the basis of which evidences D-glucose was assigned the following structure?
\[\begin{array}{cc}
\ce{CHO}\\
|\phantom{....}\\
\phantom{..}\ce{(CHOH)4}\\
|\phantom{....}\\
\phantom{..}\ce{CH2OH}
\end{array}\]
What happens when D-glucose is treated with the following reagent?
HI
Account for the following:
What happens when D – glucose is treated with the following reagents
HNO3
Glucose with excess of phenyl hydrazine forms ______.
Give a reason for the following observations:
Penta-acetate of glucose does not react with hydroxylamine.
