Advertisements
Advertisements
Question
On the basis of which evidences D-glucose was assigned the following structure?
\[\begin{array}{cc}
\ce{CHO}\\
|\phantom{....}\\
\phantom{..}\ce{(CHOH)4}\\
|\phantom{....}\\
\phantom{..}\ce{CH2OH}
\end{array}\]
Solution
This structure was assigned on the basis of the following evidences:
1. Molecular formula: The molecular formula of glucose has been found to be \[\ce{C6H12O6}\].
2. Straight chain structure:
(i) When aqueous solution of glucose is treated with sodium amalgam (Na/Hg) or sodium borohydride, it is reduced to sorbitol (or glucitol) a hexahedric alcohol.
\[\begin{array}{cc}
\phantom{.}\ce{CHO}\phantom{.......................}\ce{CH2OH}\phantom{..}\\
\phantom{}|\phantom{...........................}|\phantom{........}\\
\ce{(CHOH)4 + 2[H] ->[Na amalgam] (CHOH)4}\\
\phantom{}|\phantom{...........................}|\phantom{........}\\\
\phantom{..}\ce{CH2OH}\phantom{....................}\ce{\underset{Sorbitol}{CH2OH}\phantom{....}}
\end{array}\]
(ii) Prolonged heating with hydriodic acid and red phosphorus at 100°C gives a mixture of n-hexane and 2-iodohexane.
\[\begin{array}{cc}
\ce{\underset{Glucose}{CH2OH(CHOH)4CHO} ->[Hl][red P, 100°C] \underset{n-hexane}{CH3(CH2)4CH3} + CH3CH(CH2)3CH3}\\
\phantom{............................................}|\\
\phantom{............................................}\ce{\underset{2-Iodohexane}{I}}
\end{array}\]
The formation of n-hexane suggests that all the six carbon atoms in glucose are arranged in a straight chain structure of glucose.
3. Presence of five hydroxyl (-OH) groups: On acetylation with acetic anhydride, glucose gives a pentaacetate. This confirms that glucose contains five –OH groups. We know that the presence of two or more –OH groups on the same carbon atom makes the molecules unstable. Now since glucose is a stable compound, therefore, the five -OH groups must present on different carbon atoms.
4. Presence of one primary alcoholic group: On oxidation with cone, nitric acid, both glucose and gluconic acid give the same dicarboxylic acid, saccharic acid or glucaric acid. The primary alcoholic group \[\ce{(CH2OH)}\] is always present at the end of the carbon chain.
5. Presence of an aldehyde (-CHO) group: Glucose reacts with hydroxylamine, \[\ce{NH2OH}\] to form glucose CHO oxime. Which suggest that glucose contains a carbonyl \[\ce{(CHOH)4}\] (>C = O) groups.
APPEARS IN
RELATED QUESTIONS
Write the product when D-glucose reacts with conc. HNO3.
Write the reactions involved when D-glucose is treated with the following reagent:
Br2 water
Write the reactions involved when D-glucose is treated with the following reagent:
(CH3CO)2O
The spatial arrangement of the given molecule is denoted by:

Choose the appropriate answer(s) for the below representation from the options given
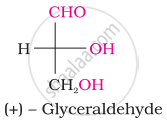
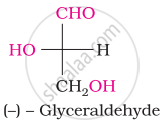
Which one of the following compounds is different from the rest?
Which of the following reactions of glucose can be explained only by its cyclic structure?
Which one of the following reactions is not explained by the open chain Structure of glucose?
Account for the following:
What happens when D – glucose is treated with the following reagents
Bromine water
Consider the following reactions:
(i) \[\ce{Glucose + R-OH ->[Conc. HNO3] [A] ->[X eq of][(CH3CO)2O] Acetyl derivative}\]
(ii) \[\ce{Glucose ->[Ni/H2] [A] ->[Y eq of][(CH3CO)2O] Acetyl derivative}\]
(iii) \[\ce{Glucose ->[Z eq of][(CH3CO)2O] Acetyl derivative}\]
'X, 'Y' and 'Z' in these reactions are respectively:
