Advertisements
Advertisements
Question
Which of the following reactions of glucose can be explained only by its cyclic structure?
Options
Glucose forms pentaacetate
Glucose reacts with hydroxylamine to form an oxime
Pentaacetate of glucose does not react with hydroxylamine
Glucose is oxidised by nitric acid to gluconic acid
Solution
Pentaacetate of glucose does not react with hydroxylamine
Explanation:
The pentaacetate of glucose does not react with hydroxylamine indicating the absence of free –CHO group. This property of glucose can be explained only by its cyclic structure.
APPEARS IN
RELATED QUESTIONS
What happens when glucose is treated with hydroxylamine?
What happens when glucose is treated with hydrogen cyanide?
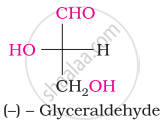
The number of asymmetric carbon atom(s) below the figure is/are

Oxime is formed by treating glucose with ____________.
The two forms of D-glucopyranose obtained from the solution of D-glucose are called ____________.
How will you distinguish 1° and 2° hydroxyl groups present in glucose? Explain with reactions.
What happens when D-glucose is treated with the following reagent?
HI
Account for the following:
What happens when D – glucose is treated with the following reagents
HNO3
Consider the following reactions:
(i) \[\ce{Glucose + R-OH ->[Conc. HNO3] [A] ->[X eq of][(CH3CO)2O] Acetyl derivative}\]
(ii) \[\ce{Glucose ->[Ni/H2] [A] ->[Y eq of][(CH3CO)2O] Acetyl derivative}\]
(iii) \[\ce{Glucose ->[Z eq of][(CH3CO)2O] Acetyl derivative}\]
'X, 'Y' and 'Z' in these reactions are respectively:
Glucose with excess of phenyl hydrazine forms ______.
