Advertisements
Advertisements
प्रश्न
Which of the following reactions of glucose can be explained only by its cyclic structure?
विकल्प
Glucose forms pentaacetate
Glucose reacts with hydroxylamine to form an oxime
Pentaacetate of glucose does not react with hydroxylamine
Glucose is oxidised by nitric acid to gluconic acid
उत्तर
Pentaacetate of glucose does not react with hydroxylamine
Explanation:
The pentaacetate of glucose does not react with hydroxylamine indicating the absence of free –CHO group. This property of glucose can be explained only by its cyclic structure.
APPEARS IN
संबंधित प्रश्न
How many moles of acetic anhydride will be required to form glucose pentaacetate from 2M of glucose?
(a) 2
(b) 5
(c) 10
(d) 2.5
Write the reactions involved when D-glucose is treated with the following reagent:
H2N-OH
The spatial arrangement of the given molecule is denoted by:

The number of asymmetric carbon atom(s) below the figure is/are

When glucose reacts with bromine water, the main product is ____________.
Which of the following properties of glucose cannot be explained by its open chain structure?
(i) Glucose does not form hydrogen sulphite with NaHSO3.
(ii) On oxidation with HNO3 glucose gives saccharic acid.
(iii) Glucose is found to exist in two different crystalline forms which are named as α and β.
A solution of D-glucose in water rotates the plane polarised light ____________.
In the following reaction, identify A and B:
\[\begin{array}{cc}
\ce{C6H12O6 ->[Acetic anhydride] A}\\
\downarrow \text{Conc. nitric acid}\phantom{...}\\
\ce{B}\phantom{.................}\end{array}\]
Why does compound (A) given below not form an oxime?
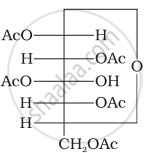
(A)
Consider the following reactions:
(i) \[\ce{Glucose + R-OH ->[Conc. HNO3] [A] ->[X eq of][(CH3CO)2O] Acetyl derivative}\]
(ii) \[\ce{Glucose ->[Ni/H2] [A] ->[Y eq of][(CH3CO)2O] Acetyl derivative}\]
(iii) \[\ce{Glucose ->[Z eq of][(CH3CO)2O] Acetyl derivative}\]
'X, 'Y' and 'Z' in these reactions are respectively:
