Advertisements
Advertisements
Question
The stability order for carbocation is _______.
(A) 2° > 3° > 1°
(B) 3° > 2° > 1°
(C) 3° > 1° > 2°
(D) 1° > 3° > 2°
Solution
(B) 3° > 2° > 1°
APPEARS IN
RELATED QUESTIONS
Out of 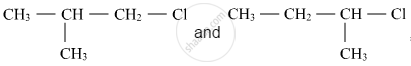 , which is more reactive towards SN1 reaction and why?
, which is more reactive towards SN1 reaction and why?
Write the structure of the major organic product in the following reaction:
\[\ce{CH3CH2Br + KCN ->[aq.ethanol]}\]
Arrange the compounds of the following set in order of reactivity towards SN2 displacement:
1-Bromobutane, 1-Bromo-2, 2-dimethylpropane, 1-Bromo-2-methylbutane, 1-Bromo-3-methylbutane
What happens when ethyl chloride is treated with aqueous KOH?
How the following conversion can be carried out?
Ethyl chloride to propanoic acid
SN1 reactions are accompanied by racemization in optically active alkyl halides.
Given reasons: SN1 reactions are accompanied by racemization in optically active alkyl halides.
Which would undergo SN2 reaction faster in the following pair and why ?
CH3 – CH2 – Br and CH3 – CH2 – I
Answer the following question.
Write one stereochemical difference between SN1 and SN2 reactions.
Arrange the following organic compounds in descending order of their reactivity towards SN1 reaction.
C6H5CH2Br, C6H5CH(C6H5)Br, C6H5CH(CH3)Br, C6H5C(CH3)(C6H5)Br
Which of the following is an example of SN2 reaction?
Which of the following is a primary halide?
SN2 mechanism proceeds through intervention of ____________.
Which of the following is the correct order of decreasing SN2 reactivity?
Which of the following compounds is optically active?
Racemic compound has ____________.
2-Bromopentane is heated with potassium ethoxide in ethanol. The major product obtained is ____________.
Which of the following undergoes nucleophilic substitution exclusively by SN1 mechanism?
The correct order of increasing the reactivity of C–X bond towards nucleophile in following compounds.
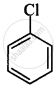
(I)
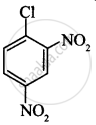
(II)
(CH3)3CCl
(III)
(CH3)2CHCl
(IV)
Which of the following compound will undergo racemisation when reacts with aq. KOH?
(i)
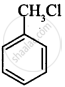
(ii)
CH3CH2CH2Cl
(iii)
\[\begin{array}{cc}
\ce{CH3}\phantom{..}\\
|\phantom{....}\\
\ce{CH3-CH-CH2Cl}
\end{array}\]
(iv)
\[\begin{array}{cc}
\phantom{..}\ce{H}\\
\phantom{..}|\\
\ce{CH3-C-Cl}\\
\phantom{..}|\\
\phantom{.....}\ce{C2H5}
\end{array}\]
Which of the following alkyl halides will undergo SN1 reaction most readily?
Write the structures and names of the compounds formed when compound ‘A’ with molecular formula, \[\ce{C7H8}\] is treated with \[\ce{Cl2}\] in the presence of \[\ce{FeCl3}\].
Cyanide ion acts as an ambident nucleophile. From which end it acts as a stronger nucleophile in aqueous medium? Give reason for your answer.
Which of the following is the definition of chirality?
CCl4 is insoluble in water because:-
Which of the following compounds will show retention in configuration on nucleophile substitution by OH− ion?
In SN1 reactions, the correct order of reactivity for the following compounds:
CH3Cl, CH3CH2Cl, (CH3)2CHCl and (CH3)3CCl is ______.
Assertion (A) : Nucleophilic substitution of iodoethane is easier than chloroethane.
Reason (R) : Bond enthalpy of C-I bond is less than that of C-Cl bond.
An organic compound A with the molecular formula (+) C4H9Br undergoes hydrolysis to form (+) C4H9OH. Give the structure of A and write the mechanism of the reaction.
Acetic anhydride from acetic acid
