Advertisements
Advertisements
Question
Which would undergo SN2 reaction faster in the following pair and why ?
CH3 – CH2 – Br and CH3 – CH2 – I
Solution
CH3 – CH2 – I would undergo an SN2 reaction faster than CH3 – CH2 – Br. Since iodine is a better leaving group because of its large size, it will be released at a faster rate in the presence of an incoming nucleophile as compared to bromine.
APPEARS IN
RELATED QUESTIONS
Write the structure of the major organic product in the following reaction:
\[\ce{CH3CH(Br)CH2CH3 + NaOH ->[water]}\]
Out of C6H5CH2Cl and C6H5CHClC6H5, which is more easily hydrolysed by aqueous KOH.
In a coordination entity of the type [PtCl2(en)2]2+ which isomer will show optical isomerism?
Which of the following reactions is an example of nucleophilic substitution reaction?
Which of the following is an optically active compound?
Identify X and Y in the following sequence:
\[\ce{C2H5Br ->[X] Product ->[Y] C3H7NH2}\]
Which of the following statements are correct about this reaction?
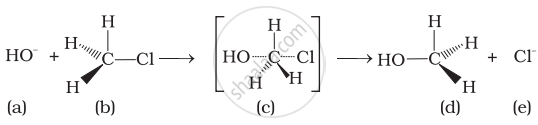
(i) The given reaction follows SN2 mechanism.
(ii) (b) and (d) have opposite configuration.
(iii) (b) and (d) have same configuration.
(iv) The given reaction follows SN1 mechanism.
Elimination reactions (especially β-elimination) are as common as the nucleophilic substitution reaction in case of alkyl halides. Specify the reagents used in both cases.
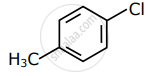
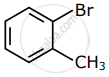
Among the following compounds I - IV, which one forms a yellow precipitate on reacting sequentially with (i) NaOH (ii) dil. HNO3 (iii) AgNO3?
 |
 |
 |
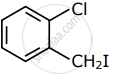 |
| I | II | III | IV |
Complete the reaction with the main product formed:

