Advertisements
Advertisements
Question
Write the structure of the major organic product in the following reaction:
\[\ce{CH3CH(Br)CH2CH3 + NaOH ->[water]}\]
Solution
\[\begin{array}{cc}
\ce{CH3 - CH - CH2CH3 + NaOH ->[H2O][Hydrolysis] CH3 - CH - CH2CH3 + NaBr + H2O}\\
|\phantom{........................................}|\phantom{......................}\\
\ce{\underset{2-Bromobutane}{Br}}\phantom{................................}\ce{\underset{Butan-2-ol}{OH}}\phantom{......................}
\end{array}\]
APPEARS IN
RELATED QUESTIONS
Write the main products when methyl chloride is treated with AgCN.
Given reasons: C–Cl bond length in chlorobenzene is shorter than C–Cl bond length in CH3–Cl.
Which alkyl halide from the following pair would you expect to react more rapidly by an SN2 mechanism? Explain your answer.
CH3CH2CH2CH2Br or \[\begin{array}{cc}
\ce{CH3CH2CHCH3}\\
\phantom{...}|\\
\phantom{....}\ce{Br}\
\end{array}\]
What is the action of the following on ethyl bromide
alcoholic solution of potassium hydroxide.
What is the action of the following on ethyl bromide:
silver acetate
Which one is most reactive towards SN1 reaction?
The order of reactivity of the given haloalkanes towards nucleophile is:
Tertiary alkyl halides are practically inert to substitution by SN2 mechanism because of ____________.
Which of the following compounds is optically active?
Identify X and Y in the following sequence:
\[\ce{C2H5Br ->[X] Product ->[Y] C3H7NH2}\]
Assertion: KCN reacts with methyl chloride to give methyl isocyanide.
Reason: CN– is an ambident nucleophile.
Which of the following statements are correct about the kinetics of this reaction?
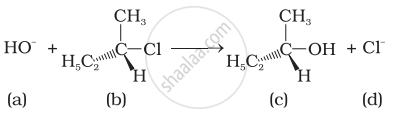
(i) The rate of reaction depends on the concentration of only (b).
(ii) The rate of reaction depends on concentration of both (a) and (b).
(iii) Molecularity of reaction is one.
(iv) Molecularity of reaction is two.
Which of the compounds will react faster in SN1 reaction with the –OH ion?
\[\ce{CH3-CH2-Cl}\] or \[\ce{C6H5-CH2-Cl}\]
Which one is the correct order of nucleophilic strength (pKa) of following nucleophiles?
The number of chiral alcohol (s) with molecular formula C4H10O is ______.
Retention of configuration is observed in ______.
Complete the reaction with the main product formed:

Give the mechanism of the following reaction:
\[\ce{CH3CH2OH ->[H2SO4][413 K] CH3CH2-O-CH2CH3 + H2O}\]
Which alkyl halide from the following pair would you expect to react more rapidly by an SN2 mechanism? Explain your answer.
\[\begin{array}{cc}\ce{CH3CHCH2CH2Br}\\|\phantom{.........}\\\ce{CH3}\phantom{......}\end{array}\] or \[\begin{array}{cc}\ce{CH3CH2CHCH2Br}\\\phantom{}|\\\phantom{...}\ce{CH3}\end{array}\]
