Advertisements
Advertisements
Question
Write the main products when methyl chloride is treated with AgCN.
Solution
When methyl chloride is treated with AgCN, methyl cyanide is formed.

APPEARS IN
RELATED QUESTIONS
Given reasons: C–Cl bond length in chlorobenzene is shorter than C–Cl bond length in CH3–Cl.
Write the structures of A, B and C in the following:

In the following pair of halogen compounds, which compound undergoes a faster SN1 reaction?
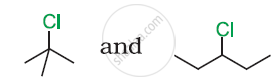
Which compound in the following pair will react faster in SN2 reaction with OH−?
CH3Br or CH3I
What is the action of the following on ethyl bromide:
moist silver oxide
The reaction of C6H5–CH=CH–CH3 with HBr produces:
Which of the following is the definition of chirality?
When CH3CH2CHCl2 is treated NaNH2 product formed is:-
Give reason for the following:
The product formed during SN1 reaction is a racemic mixture.
The following questions are case-based questions. Read the passage carefully and answer the questions that follow:
|
Nucleophilic Substitution: Influences of solvent polarity: The reaction rate (SN2) of 2-bromopropane and NaOH in ethanol containing 40% water is twice slower than in absolute ethanol. Hence the level of solvent polarity has an influence on both SN1 and SN2 reactions but with different results. Generally speaking, a weak polar solvent is favourable for SN2 reaction, while a strong polar solvent is favourable for SN1. Generally speaking, the substitution reaction of tertiary haloalkane is based on SN1 mechanism in solvents with a strong polarity (for example ethanol containing water). |
Answer the following questions:
(a) Why racemisation occurs in SN1? (1)
(b) Why is ethanol less polar than water? (1)
(c) Which one of, the following in each pair is more reactive towards SN2 reaction? (2)
(i) CH3 – CH2 – I or CH3CH2 – Cl
(ii)
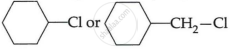
OR
(c) Arrange the following in the increasing order of their reactivity towards SN1 reactions: (2)
(i) 2-Bromo-2-methylbutane, 1-Bromo-pentane, 2-Bromo-pentane
(ii) 1-Bromo-3-methylbutane, 2-Bromo-2-methylbutane, 2-Bromo-3- methylbutane
