Advertisements
Advertisements
Question
In the following pair of halogen compounds, which compound undergoes a faster SN1 reaction?
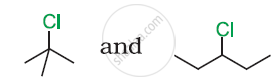
Solution
The activity of halogen compounds in SN1 reaction depends on the stability of the carbocation formed due to ionization. The order of stability is tertiary > secondary > primary. Hence, 3° alkyl chloride is more active than 2° alkyl chloride. Hence, 2° alkyl chloride is more active in SN1 reaction.
 will react faster. The carbocation
will react faster. The carbocation  will be more stable and hence the reaction will be faster.
will be more stable and hence the reaction will be faster.
APPEARS IN
RELATED QUESTIONS
Which would undergo SN1 reaction faster in the following pair and why?
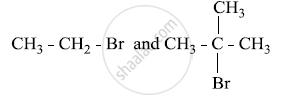
Given reasons: C–Cl bond length in chlorobenzene is shorter than C–Cl bond length in CH3–Cl.
Which would undergo SN2 reaction faster in the following pair and why ?
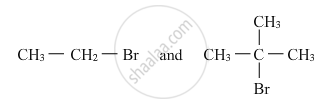
What are ambident nucleophiles? Explain with an example.
In a coordination entity of the type [PtCl2(en)2]2+ which isomer will show optical isomerism?
In the reaction, \[\ce{R - X + NaOR' -> ROR’ + X}\] ( – ve ion). The main product formed is:
Which of the following is an example of SN2 reaction?
The order of reactivities of the following alkyl halides for an SN2 reaction is:
Optically active isomers but not mirror images are called ____________.
Tertiary alkyl halides are practically inert to substitution by SN2 mechanism because of ____________.
Which of the following compounds is optically active?
Among the following, the dissociation constant is highest for:
The reaction of C6H5–CH=CH–CH3 with HBr produces:
Which of the following alkyl halides will undergo SN1 reaction most readily?
Which of the following statements are correct about the kinetics of this reaction?
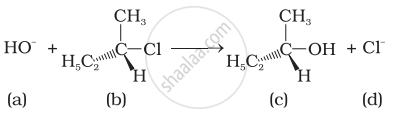
(i) The rate of reaction depends on the concentration of only (b).
(ii) The rate of reaction depends on concentration of both (a) and (b).
(iii) Molecularity of reaction is one.
(iv) Molecularity of reaction is two.
Which one is the correct order of nucleophilic strength (pKa) of following nucleophiles?
In which reaction mechanism carbocation is formed?
Which of the following compounds will show retention in configuration on nucleophile substitution by OH− ion?
Inversion of configuration occurs in ______.
Give the mechanism of the following reaction:
\[\ce{CH3CH2OH ->[H2SO4][413 K] CH3CH2-O-CH2CH3 + H2O}\]
