Advertisements
Advertisements
प्रश्न
In the following pair of halogen compounds, which compound undergoes a faster SN1 reaction?
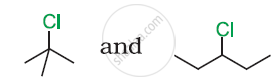
उत्तर
The activity of halogen compounds in SN1 reaction depends on the stability of the carbocation formed due to ionization. The order of stability is tertiary > secondary > primary. Hence, 3° alkyl chloride is more active than 2° alkyl chloride. Hence, 2° alkyl chloride is more active in SN1 reaction.
 will react faster. The carbocation
will react faster. The carbocation 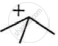 will be more stable and hence the reaction will be faster.
will be more stable and hence the reaction will be faster.
APPEARS IN
संबंधित प्रश्न
Arrange the compounds of the following set in order of reactivity towards SN2 displacement:
1-Bromo-3-methylbutane, 2-Bromo-2-methylbutane, 2-Bromo-3-methylbutane
What happens when chlorobenzene is subjected to hydrolysis?
Given reasons: SN1 reactions are accompanied by racemization in optically active alkyl halides.
What is the action of the following on ethyl bromide:
moist silver oxide
In the reaction, \[\ce{R - X + NaOR' -> ROR’ + X}\] ( – ve ion). The main product formed is:
Halogenation of alkanes is ____________.
Which of the following reactions is an example of nucleophilic substitution reaction?
An important chemical method to resolve a racemic mixture makes use of the formation of ______.
The process of separation of a racemic modification into d and l-enantiomers is called ____________.
Which of the following compound will undergo racemisation when reacts with aq. KOH?
(i)
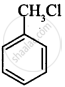
(ii)
CH3CH2CH2Cl
(iii)
\[\begin{array}{cc}
\ce{CH3}\phantom{..}\\
|\phantom{....}\\
\ce{CH3-CH-CH2Cl}
\end{array}\]
(iv)
\[\begin{array}{cc}
\phantom{..}\ce{H}\\
\phantom{..}|\\
\ce{CH3-C-Cl}\\
\phantom{..}|\\
\phantom{.....}\ce{C2H5}
\end{array}\]
Which of the following alkyl halides will undergo SN1 reaction most readily?
Assertion: KCN reacts with methyl chloride to give methyl isocyanide.
Reason: CN– is an ambident nucleophile.
Which of the compounds will react faster in SN1 reaction with the –OH ion?
\[\ce{CH3-CH2-Cl}\] or \[\ce{C6H5-CH2-Cl}\]
Compound ‘A’ with molecular formula \[\ce{C4H9Br}\] is treated with aq. \[\ce{KOH}\] solution. The rate of this reaction depends upon the concentration of the compound ‘A’ only. When another optically active isomer ‘B’ of this compound was treated with aq. \[\ce{KOH}\] solution, the rate of reaction was found to be dependent on concentration of compound and \[\ce{KOH}\] both.
(i) Write down the structural formula of both compounds ‘A’ and ‘B’.
(ii) Out of these two compounds, which one will be converted to the product with inverted configuration.
Aryl halides are extremely less reactive towards nucleophilic substitution. Predict and explain the order of reactivity of the following compounds towards nucleophilic substitution:
| (I) | 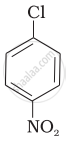 |
| (II) | 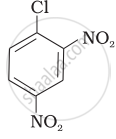 |
| (III) | 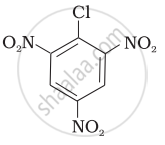 |
When CH3CH2CHCl2 is treated NaNH2 product formed is:-
CCl4 is insoluble in water because:-
Arrange the following compounds in increasing order of reactivity towards SN2 reaction.
2-Bromopentane, 1-Bromopentane, 2-Bromo-2-methylbutane
Complete the reaction with the main product formed:

Discuss SN2 mechanism of methyl bromide using aqueous KOH.
