Advertisements
Advertisements
प्रश्न
In the following pair of halogen compounds, which compound undergoes a faster SN1 reaction?
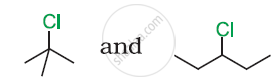
उत्तर
The activity of halogen compounds in SN1 reaction depends on the stability of the carbocation formed due to ionization. The order of stability is tertiary > secondary > primary. Hence, 3° alkyl chloride is more active than 2° alkyl chloride. Hence, 2° alkyl chloride is more active in SN1 reaction.
 will react faster. The carbocation
will react faster. The carbocation  will be more stable and hence the reaction will be faster.
will be more stable and hence the reaction will be faster.
APPEARS IN
संबंधित प्रश्न
Write the main products when methyl chloride is treated with AgCN.
Write the structure of the major product in each of the following reaction :
In the following pair of halogen compounds, which compound undergoes a faster SN1 reaction?
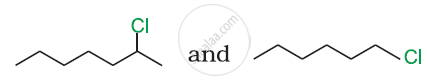
Which compound in the following pair will react faster in SN2 reaction with OH−?
(CH3)3CCl or CH3Cl
How will you bring about the following conversion?
Toluene to benzyl alcohol
Write the structure of the major organic product in the following reaction:
\[\ce{CH3CH2Br + KCN ->[aq.ethanol]}\]
Write the mechanism of the following reaction:
\[\ce{{n}BuBr + KCN ->[EtOH-H2O] {n}BuCN}\]
What happens when chlorobenzene is subjected to hydrolysis?
What happens when methyl chloride is treated with KCN?
Answer the following question.
Write one stereochemical difference between SN1 and SN2 reactions.
Which of the following reactions is an example of nucleophilic substitution reaction?
Which one of the following halogen compounds is difficult to be hydrolysed by SN1 mechanism?
The increasing order of nucleophilicity would be:
Which of the following compound will undergo racemisation when reacts with aq. KOH?
(i)
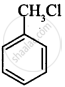
(ii)
CH3CH2CH2Cl
(iii)
\[\begin{array}{cc}
\ce{CH3}\phantom{..}\\
|\phantom{....}\\
\ce{CH3-CH-CH2Cl}
\end{array}\]
(iv)
\[\begin{array}{cc}
\phantom{..}\ce{H}\\
\phantom{..}|\\
\ce{CH3-C-Cl}\\
\phantom{..}|\\
\phantom{.....}\ce{C2H5}
\end{array}\]
The increasing order of reactivity towards SN1 mechanism is:
(I) \[\begin{array}{cc}
\ce{CH3-CH-CH2-CH3}\\
|\phantom{........}\\
\ce{CH3}\phantom{.....}
\end{array}\]
(II) CH3CH2CH2Cl
(III) P–CH3O–C6H4–CH2Cl
Write the structures and names of the compounds formed when compound ‘A’ with molecular formula, \[\ce{C7H8}\] is treated with \[\ce{Cl2}\] in the presence of \[\ce{FeCl3}\].
CCl4 is insoluble in water because:-
Give reason for the following:
The product formed during SN1 reaction is a racemic mixture.
Acetic anhydride from acetic acid
Which alkyl halide from the following pair would you expect to react more rapidly by an SN2 mechanism? Explain your answer.
\[\begin{array}{cc}\ce{CH3CH2CHCH3}\\\phantom{...}|\\\phantom{....}\ce{Br}\end{array}\] or \[\begin{array}{cc}\phantom{.....}\ce{CH3}\\\phantom{..}|\\\ce{H3C - C - Br}\\\phantom{..}|\\\phantom{....}\ce{CH3}\end{array}\]
