Advertisements
Advertisements
प्रश्न
Which compound in the following pair will react faster in SN2 reaction with OH−?
(CH3)3CCl or CH3Cl
उत्तर
\[\begin{array}{cc}
\phantom{....}\ce{CH3}\\
\phantom{..}|\\
\ce{CH3 - C - Cl}\\
\phantom{..}|\\
\phantom{....}\ce{CH3}\\
\end{array}\] or CH3 — Cl
The SN2 mechanism involves the attack of the nucleophile at the atom bearing the leaving group. But, in case of (CH3)3CCl, the attack of the nucleophile at the carbon atom is hindered because of the presence of bulky substituents on that carbon atom bearing the leaving group. On the other hand, there are no bulky substituents on the carbon atom bearing the leaving group in CH3Cl. Hence, CH3Cl reacts faster than (CH3)3CCl in SN2 reaction with OH−.
APPEARS IN
संबंधित प्रश्न
Write the structures of A, B and C in the following:

Write the structure of the major organic product in the following reaction:
\[\ce{CH3CH2Br + KCN ->[aq.ethanol]}\]
How the following conversion can be carried out?
Ethyl chloride to propanoic acid
Given reasons: The dipole moment of chlorobenzene is lower than that of cyclohexyl chloride.
What is the action of the following on ethyl bromide:
silver acetate
Answer the following question.
Write one stereochemical difference between SN1 and SN2 reactions.
In the reaction, \[\ce{R - X + NaOR' -> ROR’ + X}\] ( – ve ion). The main product formed is:
Which compound in the following pair reacts faster in SN2 reaction with OH–?
- CH3Br or CH3
- CH3Cl, (CH3)3CCl
An important chemical method to resolve a racemic mixture makes use of the formation of ______.
SN1 reaction of alkyl halides lead to ___________.
Identify the end product (C) in the following sequence:
\[\ce{C2H5OH ->[SOCl2][Pyridine] A ->[KCN {(alc.)}] B ->[2H2O/H^+] C}\]
Identify X and Y in the following sequence:
\[\ce{C2H5Br ->[X] Product ->[Y] C3H7NH2}\]
Complete the following analogy:
Same molecular formula but different structures: A : : Non superimposable mirror images: B
Read the passage given below and answer the following question:
Nucleophilic substitution reaction of haloalkane can be conducted according to both SN1 and SN2 mechanisms. However, which mechanism it is based on is related to such factors as the structure of haloalkane, and properties of leaving group, nucleophilic reagent and solvent.
Influences of halogen: No matter which mechanism the nucleophilic substitution reaction is based on, the leaving group always leave the central carbon atom with electron pair. This is just the opposite of the situation that nucleophilic reagent attacks the central carbon atom with electron pair. Therefore, the weaker the alkalinity of leaving group is, the more stable the anion formed is and it will be more easier for the leaving group to leave the central carbon atom; that is to say, the reactant is more easier to be substituted. The alkalinity order of halogen ion is I− < Br− < Cl− < F− and the order of their leaving tendency should be I− > Br− > Cl− > F−. Therefore, in four halides with the same alkyl and different halogens, the order of substitution reaction rate is RI > RBr > RCl > RF. In addition, if the leaving group is very easy to leave, many carbocation intermediates are generated in the reaction and the reaction is based on SN1 mechanism. If the leaving group is not easy to leave, the reaction is based on SN2 a mechanism.
Influences of solvent polarity: In SN1 reaction, the polarity of the system increases from the reactant to the transition state, because polar solvent has a greater stabilizing effect on the transition state than the reactant, thereby reduce activation energy and accelerate the reaction. In SN2 reaction, the polarity of the system generally does not change from the reactant to the transition state and only charge dispersion occurs. At this time, polar solvent has a great stabilizing effect on Nu than the transition state, thereby increasing activation energy and slow down the reaction rate. For example, the decomposition rate (SN1) of tertiary chlorobutane in 25℃ water (dielectric constant 79) is 300000 times faster than in ethanol (dielectric constant 24). The reaction rate (SN2) of 2-bromopropane and NaOH in ethanol containing 40% water is twice slower than in absolute ethanol. In a word, the level of solvent polarity has influence on both SN1 and SN2 reactions, but with different results. Generally speaking, weak polar solvent is favorable for SN2 reaction, while strong polar solvent is favorable for SN1 reaction, because only under the action of polar solvent can halogenated hydrocarbon dissociate into carbocation and halogen ion and solvents with a strong polarity is favorable for solvation of carbocation, increasing its stability. Generally speaking, the substitution reaction of tertiary haloalkane is based on SN1 mechanism in solvents with a strong polarity (for example, ethanol containing water).
SN1 reaction will be fastest in which of the following solvents?
A primary alkyl halide would prefer to undergo ______.
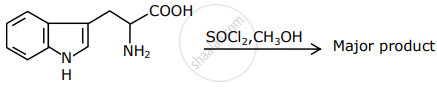
The major product formed in the following reaction is:
Racemisation occurs in ______.
Convert bromoethane to propanamine.
Acetic anhydride from acetic acid
