Advertisements
Advertisements
प्रश्न
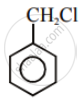
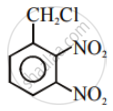
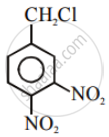
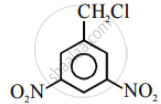
In the following pair of halogen compounds, which compound undergoes a faster SN1 reaction?
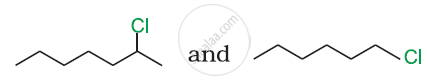
उत्तर
The activity of halogen compounds in SN1 reaction depends on the stability of the carbocation formed due to ionization. The order of stability is tertiary > secondary > primary. Hence, 2° alkyl chloride is more active than 1° alkyl chloride. Hence, 2° alkyl chloride is more active in SN1 reaction.
 reacts faster due to the greater stability of 2° carbocation than 1° carbocation.
reacts faster due to the greater stability of 2° carbocation than 1° carbocation.
APPEARS IN
संबंधित प्रश्न
Give reasons for the following:
(CH3)3C–O–CH3 on reaction with HI gives (CH3)3C–I and CH3–OH as the main products and not (CH3)3C–OH and CH3–I.
Out of 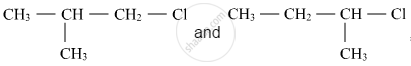 , which is more reactive towards SN1 reaction and why?
, which is more reactive towards SN1 reaction and why?
Given reasons: C–Cl bond length in chlorobenzene is shorter than C–Cl bond length in CH3–Cl.
How will you bring about the following conversion?
Toluene to benzyl alcohol
Arrange the compounds of the following set in order of reactivity towards SN2 displacement:
1-Bromobutane, 1-Bromo-2, 2-dimethylpropane, 1-Bromo-2-methylbutane, 1-Bromo-3-methylbutane
Out of C6H5CH2Cl and C6H5CHClC6H5, which is more easily hydrolysed by aqueous KOH.
Which one of the following halogen compounds is difficult to be hydrolysed by SN1 mechanism?
Isopropyl chloride undergoes hydrolysis by:
Tertiary alkyl halides are practically inert to substitution by SN2 mechanism because of ____________.
Which of the following is an optically active compound?
The process of separation of a racemic modification into d and l-enantiomers is called ____________.
SN1 reaction of alkyl halides lead to ___________.
Which of the following alkyl halides will undergo SN1 reaction most readily?
Which of the following statements are correct about this reaction?
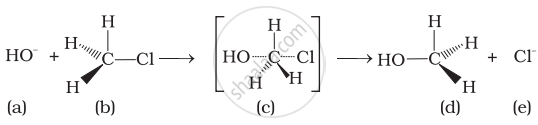
(i) The given reaction follows SN2 mechanism.
(ii) (b) and (d) have opposite configuration.
(iii) (b) and (d) have same configuration.
(iv) The given reaction follows SN1 mechanism.
In SN1 reactions, the correct order of reactivity for the following compounds:
CH3Cl, CH3CH2Cl, (CH3)2CHCl and (CH3)3CCl is ______.
The decreasing order of reactivity of the following compounds towards nucleophilic substitution (SN2) is ______.
Complete the reaction with the main product formed:

Convert bromoethane to propanamine.