Advertisements
Advertisements
प्रश्न
Out of C6H5CH2Cl and C6H5CHClC6H5, which is more easily hydrolysed by aqueous KOH.
उत्तर
C6H5CHClC6H5 is hydrolysed faster.
- The hydrolysis of an alkyl halide is a nucleophilic substitution reaction. For aryl halides, this occurs through the SN1 method, resulting in carbocation.
- C6H5CH2Cl or benzyl chloride gives
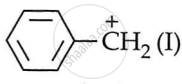 carbocation while C6H5CHClC6H5 generates
carbocation while C6H5CHClC6H5 generates 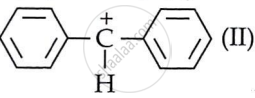 .
. - Out of I and II, carbocation II is more stable. Two attached phenyl rings on the carbon carry the positive charge.
- This leads to increased delocalisation of the positive charge and greater carbocation stability. This leads to faster formation of (II) and easier hydrolysis of the resulting halide than benzyl chloride.
APPEARS IN
संबंधित प्रश्न
Discuss the mechanism of alkaline hydrolysis of bromomethane.
Write the structure of the major organic product in the following reaction:
\[\ce{CH3CH(Br)CH2CH3 + NaOH ->[water]}\]
Write the mechanism of the following reaction:
\[\ce{{n}BuBr + KCN ->[EtOH-H2O] {n}BuCN}\]
Arrange the compounds of the following set in order of reactivity towards SN2 displacement:
1-Bromo-3-methylbutane, 2-Bromo-2-methylbutane, 2-Bromo-3-methylbutane
Given reasons: SN1 reactions are accompanied by racemization in optically active alkyl halides.
What is the action of the following on ethyl bromide?
moist silver oxide
What is the action of the following on ethyl bromide:
silver acetate
Answer the following question.
Write one stereochemical difference between SN1 and SN2 reactions.
An important chemical method to resolve a racemic mixture makes use of the formation of ______.
Identify the end product (C) in the following sequence:
\[\ce{C2H5OH ->[SOCl2][Pyridine] A ->[KCN {(alc.)}] B ->[2H2O/H^+] C}\]
Identify X and Y in the following sequence:
\[\ce{C2H5Br ->[X] Product ->[Y] C3H7NH2}\]
Which of the following alkyl halides will undergo SN1 reaction most readily?
Which of the following compounds will give a racemic mixture on nucleophilic substitution by OH ion?
1-Bromoethane, 1-Bromopropane, 1-Bromobutane, Bromobenzene
Which of the following statements are correct about this reaction?
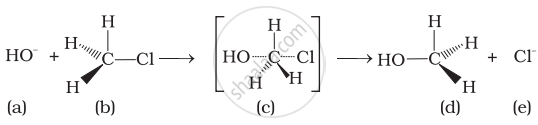
(i) The given reaction follows SN2 mechanism.
(ii) (b) and (d) have opposite configuration.
(iii) (b) and (d) have same configuration.
(iv) The given reaction follows SN1 mechanism.
Aryl halides are extremely less reactive towards nucleophilic substitution. Predict and explain the order of reactivity of the following compounds towards nucleophilic substitution:
| (I) | 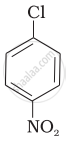 |
| (II) | 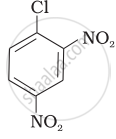 |
| (III) | 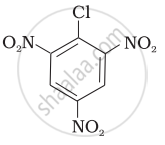 |
Which one is the correct order of nucleophilic strength (pKa) of following nucleophiles?
The number of chiral carbons present in the molecule given below is ______.
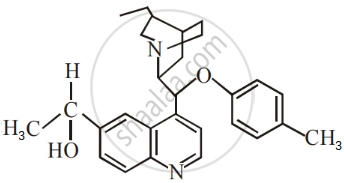
Racemisation occurs in ______.
Complete the reaction with the main product formed:

