Advertisements
Advertisements
प्रश्न
Which of the following statements are correct about this reaction?
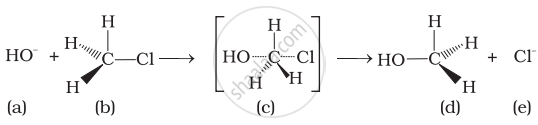
(i) The given reaction follows SN2 mechanism.
(ii) (b) and (d) have opposite configuration.
(iii) (b) and (d) have same configuration.
(iv) The given reaction follows SN1 mechanism.
उत्तर
(i) The given reaction follows SN2 mechanism.
(ii) (b) and (d) have opposite configuration.
Explanation:
In the given reaction, alkyl halide is primary in nature. Here, a transitory state is observed in which one bond is broken and one bond is formed synchronously he., in one step. So, it follows SN2 mechanism.
In this mechanism, nucleophile attacks the carbon at 180° to the leaving group. So the reactant and product have opposite configuration.
APPEARS IN
संबंधित प्रश्न
Out of 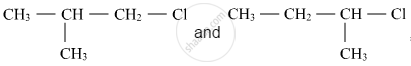 , which is more reactive towards SN1 reaction and why?
, which is more reactive towards SN1 reaction and why?
Given reasons: C–Cl bond length in chlorobenzene is shorter than C–Cl bond length in CH3–Cl.
Which compound in the following pair will react faster in SN2 reaction with OH−?
(CH3)3CCl or CH3Cl
What is the action of the following on ethyl bromide:
silver acetate
Arrange the following organic compounds in descending order of their reactivity towards SN1 reaction.
C6H5CH2Br, C6H5CH(C6H5)Br, C6H5CH(CH3)Br, C6H5C(CH3)(C6H5)Br
In the SN1 reaction, racemization takes place. It is due to:
Isopropyl chloride undergoes hydrolysis by:
Tertiary alkyl halides are practically inert to substitution by SN2 mechanism because of ____________.
The correct order of increasing the reactivity of C–X bond towards nucleophile in following compounds.
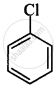
(I)
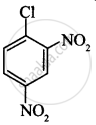
(II)
(CH3)3CCl
(III)
(CH3)2CHCl
(IV)
Match the reactions given in Column I with the types of reactions given in Column II.
| Column I | Column II | |
| (i) | 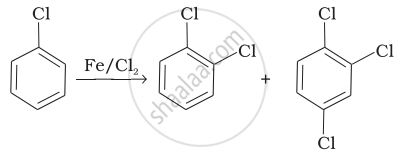 |
(a) Nucleophilic aromatic substitution |
| (ii) | \[\begin{array}{cc} \ce{CH3 - CH = CH2 + HBr -> CH3 - CH - CH3}\\ \phantom{............................}|\phantom{}\\ \phantom{.............................}\ce{Br}\phantom{} \end{array}\] |
(b) Electrophilic aromatic substitution |
| (iii) | 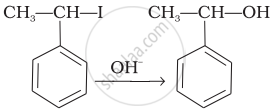 |
(c) Saytzeff elimination |
| (iv) | 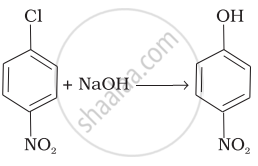 |
(d) Electrophilic addition |
| (v) | \[\begin{array}{cc} \ce{CH3 CH2 CH CH3 ->[alc.KOH] CH3 CH = CH CH3}\\ \phantom{}|\phantom{..........................}\\ \phantom{}\ce{Br}\phantom{........................} \end{array}\] |
(e) Nucleophilic substitution (SN1) |
