Advertisements
Advertisements
प्रश्न
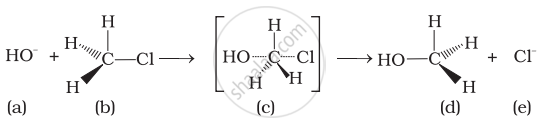
Which of the statements are correct about above reaction?
(i) (a) and (e) both are nucleophiles.
(ii) In (c) carbon atom is sp3 hybridised.
(iii) In (c) carbon atom is sp2 hybridised.
(iv) (a) and (e) both are electrophiles.
उत्तर
(i) (a) and (e) both are nucleophiles.
(iii) In (c) carbon atom is sp2 hybridised.
Explanation:
HO− and Cl− are nucleophiles.
In (iii), C atom is sp2 hybridised due to formation of \[\ce{C - OH}\] bond and breaking of \[\ce{C - Cl}\] bond simultaneously. So, in the transition state, the C atom is bonded to only 3 H atoms completely.
APPEARS IN
संबंधित प्रश्न
Write the isomers of the compound having the formula C4H9Br.
Which compound in the following pair will react faster in SN2 reaction with OH−?
CH3Br or CH3I
Write the structure of the major organic product in the following reaction:
\[\ce{CH3CH(Br)CH2CH3 + NaOH ->[water]}\]
What is the action of the following on ethyl bromide
alcoholic solution of potassium hydroxide.
Which of the following compounds is optically active?
The increasing order of nucleophilicity would be:
The reaction of C6H5–CH=CH–CH3 with HBr produces:
Which of the compounds will react faster in SN1 reaction with the –OH ion?
\[\ce{CH3-CH2-Cl}\] or \[\ce{C6H5-CH2-Cl}\]
When CH3CH2CHCl2 is treated NaNH2 product formed is:-
Which one of the following compounds is more reactive towards SN1 reaction?
