Advertisements
Advertisements
प्रश्न
Which compound in the following pair will react faster in SN2 reaction with OH−?
CH3Br or CH3I
उत्तर
In the SN2 mechanism, the reactivity of halides for the same alkyl group increases in the order. This happens because as the size increases, the halide ion becomes a better leaving group.
R−F << R−Cl < R−Br < R−I
Therefore, CH3I will react faster than CH3Br in SN2 reactions with OH−.
APPEARS IN
संबंधित प्रश्न
Write the major products(s) in the following:
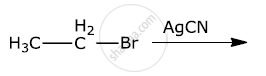
Which would undergo SN2 reaction faster in the following pair and why ?
Out of C6H5CH2Cl and C6H5CHClC6H5, which is more easily hydrolysed by aqueous KOH.
SN1 reactions are accompanied by racemization in optically active alkyl halides.
Arrange the following organic compounds in descending order of their reactivity towards SN1 reaction.
C6H5CH2Br, C6H5CH(C6H5)Br, C6H5CH(CH3)Br, C6H5C(CH3)(C6H5)Br
Which of the following is optically inactive?
Which of the following reactions is an example of nucleophilic substitution reaction?
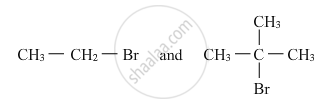
The order of reactivities of the following alkyl halides for an SN2 reaction is:
Which of the following is the correct order of decreasing SN2 reactivity?
Identify the end product (C) in the following sequence:
\[\ce{C2H5OH ->[SOCl2][Pyridine] A ->[KCN {(alc.)}] B ->[2H2O/H^+] C}\]
Identify X and Y in the following sequence:
\[\ce{C2H5Br ->[X] Product ->[Y] C3H7NH2}\]
Which of the following undergoes nucleophilic substitution exclusively by SN1 mechanism?
The increasing order of nucleophilicity would be:
The correct order of increasing the reactivity of C–X bond towards nucleophile in following compounds.
(I)
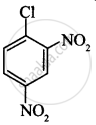
(II)
(CH3)3CCl
(III)
(CH3)2CHCl
(IV)
Which of the following statements are correct about this reaction?
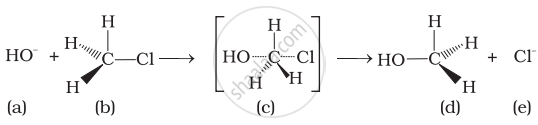
(i) The given reaction follows SN2 mechanism.
(ii) (b) and (d) have opposite configuration.
(iii) (b) and (d) have same configuration.
(iv) The given reaction follows SN1 mechanism.
Elimination reactions (especially β-elimination) are as common as the nucleophilic substitution reaction in case of alkyl halides. Specify the reagents used in both cases.
Cyanide ion acts as an ambident nucleophile. From which end it acts as a stronger nucleophile in aqueous medium? Give reason for your answer.
Chlorination of alkanes is an example of
The number of chiral alcohol (s) with molecular formula C4H10O is ______.
The number of chiral carbons present in the molecule given below is ______.