Advertisements
Advertisements
प्रश्न
Cyanide ion acts as an ambident nucleophile. From which end it acts as a stronger nucleophile in aqueous medium? Give reason for your answer.
उत्तर
It acts as a stronger nucleophile from the carbon end because it will lead to the formation of C – C bond which is more stable (bond between two similar atoms) than C – N bond.
APPEARS IN
संबंधित प्रश्न
What happens when ethyl chloride is treated with aqueous KOH?
Given reasons: The dipole moment of chlorobenzene is lower than that of cyclohexyl chloride.
Which of the following reactions is an example of nucleophilic substitution reaction?
Most reactive halide towards SN1 reaction is ____________.
Compound ‘A’ with molecular formula \[\ce{C4H9Br}\] is treated with aq. \[\ce{KOH}\] solution. The rate of this reaction depends upon the concentration of the compound ‘A’ only. When another optically active isomer ‘B’ of this compound was treated with aq. \[\ce{KOH}\] solution, the rate of reaction was found to be dependent on concentration of compound and \[\ce{KOH}\] both.
(i) Write down the structural formula of both compounds ‘A’ and ‘B’.
(ii) Out of these two compounds, which one will be converted to the product with inverted configuration.
How do polar solvents help in the first step in SN1 mechanism?
Give reason for the following:
The product formed during SN1 reaction is a racemic mixture.
The number of chiral carbons present in the molecule given below is ______.
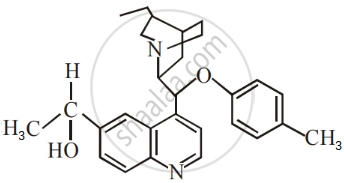
Optical activity of an enantiomeric mixture is +12.6° and the specific rotation of (+) isomer is +30°. The optical purity is ______ %.
Discuss the mechanism of alkaline hydrolysis of methyl bromide.
