Advertisements
Advertisements
प्रश्न
Give reason for the following:
The product formed during SN1 reaction is a racemic mixture.
उत्तर
During the SN1 mechanism, intermediate carbocation formed is sp2 hybridized and planar in nature. This allows the attack of nucleophiles from either side of the plane resulting in a racemic mixture.
APPEARS IN
संबंधित प्रश्न
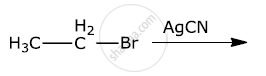
Write the major products(s) in the following:
Given reasons: C–Cl bond length in chlorobenzene is shorter than C–Cl bond length in CH3–Cl.
Write the structures of A, B and C in the following:

In the following pair of halogen compounds, which compound undergoes a faster SN1 reaction?
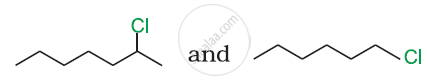
Which compound in the following pair will react faster in SN2 reaction with OH−?
CH3Br or CH3I
Among the following, the dissociation constant is highest for:
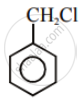
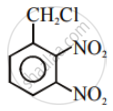
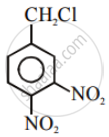
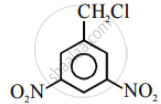
Aryl halides are extremely less reactive towards nucleophilic substitution. Predict and explain the order of reactivity of the following compounds towards nucleophilic substitution:
| (I) | 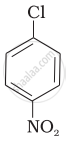 |
| (II) | 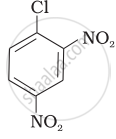 |
| (III) | 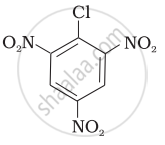 |
The decreasing order of reactivity of the following compounds towards nucleophilic substitution (SN2) is ______.
Give the mechanism of the following reaction:
\[\ce{CH3CH2OH ->[H2SO4][413 K] CH3CH2-O-CH2CH3 + H2O}\]
Acetic anhydride from acetic acid