Advertisements
Advertisements
Question
Give reason for the following:
The product formed during SN1 reaction is a racemic mixture.
Solution
During the SN1 mechanism, intermediate carbocation formed is sp2 hybridized and planar in nature. This allows the attack of nucleophiles from either side of the plane resulting in a racemic mixture.
APPEARS IN
RELATED QUESTIONS
Discuss the mechanism of alkaline hydrolysis of bromomethane.
Give reasons for the following:
(CH3)3C–O–CH3 on reaction with HI gives (CH3)3C–I and CH3–OH as the main products and not (CH3)3C–OH and CH3–I.
Write the mechanism of the following reaction:
\[\ce{{n}BuBr + KCN ->[EtOH-H2O] {n}BuCN}\]
The stability order for carbocation is _______.
(A) 2° > 3° > 1°
(B) 3° > 2° > 1°
(C) 3° > 1° > 2°
(D) 1° > 3° > 2°
Given reasons: The dipole moment of chlorobenzene is lower than that of cyclohexyl chloride.
Given reasons: SN1 reactions are accompanied by racemization in optically active alkyl halides.
Most reactive halide towards SN1 reaction is ____________.
The process of separation of a racemic modification into d and l-enantiomers is called ____________.
The correct order of increasing the reactivity of C–X bond towards nucleophile in following compounds.
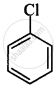
(I)
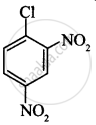
(II)
(CH3)3CCl
(III)
(CH3)2CHCl
(IV)
Retention of configuration is observed in ______.
