Advertisements
Advertisements
Question
Write the mechanism of the following reaction:
\[\ce{{n}BuBr + KCN ->[EtOH-H2O] {n}BuCN}\]
Solution 1
This reaction is a first-order nucleophilic substitution (SN1). The mechanism can be stated as:
Step 1: Generation of nucleophile:
\[\ce{KCN ->[EtOH-H2O]K+ + \overset{—}{C} ≡ N}\]
Step 2: Nucleophilic attack and formation of transition state:
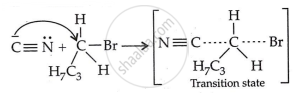
Step 3: Generation of product:
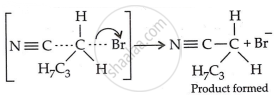
Therefore, we have
\[\ce{CH3 - CH2 - CH2 - CH2 - Br + KCN ->[EtOH/H2O]CH3CH2CH2CH2CN + KBr}\]
Solution 2
KCN is the resonating hybrid of the following structures:
\[\ce{K^+ [^- :C ≡ N: ↔ :C = \overset{\bullet\bullet}{N} :^-]}\]
Therefore, CN⁻ acts as an ambident nucleophile. It can attack the carbon atom of the C-Br bond in n-BuBr through either the carbon (C) or nitrogen (N) atom. Since the C-N bond is weaker than the C-C bond, the attack occurs at the carbon atom, leading to the formation of n-butyl cyanide.
\[\ce{K^+CN^- + \underset{n-butyl bromide}{CH3CH2CH2\overset{δ+}{C}H2 - \overset{δ-}{B}r} -> \underset{n-butyl cyanide}{CH3CH2CH2CH2CN} + KBr}\]
Notes
Students can refer to the provided solutions based on their preferred marks.
APPEARS IN
RELATED QUESTIONS
Arrange the compounds of the following set in order of reactivity towards SN2 displacement:
1-Bromo-3-methylbutane, 2-Bromo-2-methylbutane, 2-Bromo-3-methylbutane
Arrange the compounds of the following set in order of reactivity towards SN2 displacement:
1-Bromobutane, 1-Bromo-2, 2-dimethylpropane, 1-Bromo-2-methylbutane, 1-Bromo-3-methylbutane
Identify 'A' in the following reaction -
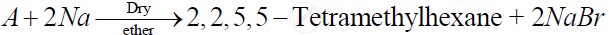
(a) 2- Bromo-2 methylbutane
(b) 1 -Bromo-2,2-dimethylpropane
(c) 1 - Bromo - 3 -methylbutane
(d) 1 - Bromo- 2 -methylpropane
What is the action of the following on ethyl bromide:
moist silver oxide
AgCN reacts with haloalkanes to form isocyanide. Haloalkanes react with KCN to form alkyl cyanides as the main product. Why?
The order of reactivities of the following alkyl halides for an SN2 reaction is:
Optically active isomers but not mirror images are called ____________.
Among the following, the dissociation constant is highest for:
SN1 reaction of alkyl halides lead to ___________.
A primary alkyl halide would prefer to undergo ______.
Which of the following statements are correct about this reaction?
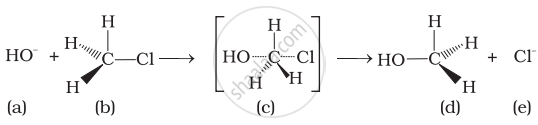
(i) The given reaction follows SN2 mechanism.
(ii) (b) and (d) have opposite configuration.
(iii) (b) and (d) have same configuration.
(iv) The given reaction follows SN1 mechanism.
Ethylene chloride and ethylidene chloride are isomers. Identify the correct statements.
(i) Both the compounds form same product on treatment with alcoholic KOH.
(ii) Both the compounds form same product on treatment with aq.NaOH.
(iii) Both the compounds form same product on reduction.
(iv) Both the compounds are optically active.
Aryl halides are extremely less reactive towards nucleophilic substitution. Predict and explain the order of reactivity of the following compounds towards nucleophilic substitution:
| (I) | 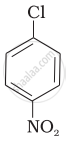 |
| (II) | 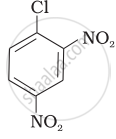 |
| (III) | 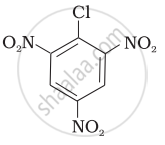 |
Which one is the correct order of nucleophilic strength (pKa) of following nucleophiles?
The number of chiral alcohol (s) with molecular formula C4H10O is ______.
Which of the following compounds will show retention in configuration on nucleophile substitution by OH− ion?
Racemisation occurs in ______.
Assertion (A) : Nucleophilic substitution of iodoethane is easier than chloroethane.
Reason (R) : Bond enthalpy of C-I bond is less than that of C-Cl bond.
Retention of configuration is observed in ______.
Convert bromoethane to propanamine.
