Advertisements
Advertisements
Question
Arrange the compounds of the following set in order of reactivity towards SN2 displacement:
2-Bromo-2-methylbutane, 1-Bromopentane, 2-Bromopentane
Solution
The SN2 reaction involves the formation of a transition state with the carbon atom surrounded by 5 additional atoms (groups). A transition state requires minimum steric interactions. The most suitable substrates for SN2 reactions are 1° alkyl halides, followed by 2° and 3° alkyl halides. The order of reactivity towards SN2 is 1° > 2° > 3°> aryl halide. Based on this, the order will be
\[\begin{array}{cc}
\phantom{.....................}\ce{Br}\phantom{...........................}\ce{Br}\\
\phantom{...................}|\phantom{.............................}|\\
\ce{\underset{1-Bromopentane}{H3C - (CH2)3 - CH2Br} > \ce{\underset{2-Bromopentane}{CH3 - CH - (CH2)2 - CH3}} > \ce{CH3 - C - CH2 - CH3}}\\
\phantom{.................................................}|\\
\phantom{....................................................}\ce{\underset{2-Brmo-2-methylbutane}{CH3}}\
\end{array}\]
APPEARS IN
RELATED QUESTIONS
Which would undergo SN1 reaction faster in the following pair and why?
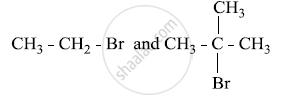
Give reasons for the following:
(CH3)3C–O–CH3 on reaction with HI gives (CH3)3C–I and CH3–OH as the main products and not (CH3)3C–OH and CH3–I.
Which compound in the following pair will react faster in SN2 reaction with OH−?
CH3Br or CH3I
Write the mechanism of the following reaction:
\[\ce{{n}BuBr + KCN ->[EtOH-H2O] {n}BuCN}\]
What happens when ethyl chloride is treated with aqueous KOH?
Which compound in the following pair reacts faster in SN2 reaction with OH–?
- CH3Br or CH3
- CH3Cl, (CH3)3CCl
Which of the following is optically inactive?
Which of the following pairs is/are correctly matched?
| Reaction | Product | |
| I | RX + AgCN | RNC |
| II | RX + KCN | RCN |
| III | RX + KNO2 | \[\begin{array}{cc} \phantom{.......}\ce{O}\\ \phantom{.....}/\\ \ce{R - N}\phantom{....}\\ \phantom{.....}\backslash\backslash\\ \phantom{.......}\ce{O} \end{array}\] |
| IV | RX + AgNO2 | \[\ce{R-O-N=O}\] |
Halogenation of alkanes is ____________.
Which one is most reactive towards SN1 reaction?
Most reactive halide towards SN1 reaction is ____________.
The order of reactivities of the following alkyl halides for an SN2 reaction is:
Which of the following is a chiral compound?
The increasing order of reactivity towards SN1 mechanism is:
(I) \[\begin{array}{cc}
\ce{CH3-CH-CH2-CH3}\\
|\phantom{........}\\
\ce{CH3}\phantom{.....}
\end{array}\]
(II) CH3CH2CH2Cl
(III) P–CH3O–C6H4–CH2Cl
How do polar solvents help in the first step in SN1 mechanism?
Aryl halides are extremely less reactive towards nucleophilic substitution. Predict and explain the order of reactivity of the following compounds towards nucleophilic substitution:
| (I) | 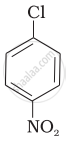 |
| (II) | 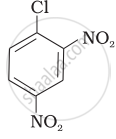 |
| (III) | 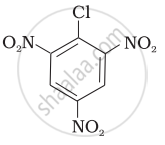 |
Arrange the following compounds in increasing order of reactivity towards SN2 reaction.
2-Bromopentane, 1-Bromopentane, 2-Bromo-2-methylbutane
Discuss SN2 mechanism of methyl bromide using aqueous KOH.
Discuss the mechanism of alkaline hydrolysis of methyl bromide.
