Advertisements
Advertisements
Question
How do polar solvents help in the first step in SN1 mechanism?
Solution
The SN1 mechanism proceeds through the formation of carbocation. It involves breaking of C-halogen bond for which energy is obtained through the salvation of halide ion with the proton of the protic solvent. Thus, polar solvents help in ionisations step by stabilizing the ions by solvation.
APPEARS IN
RELATED QUESTIONS
Which would undergo SN2 reaction faster in the following pair and why ?
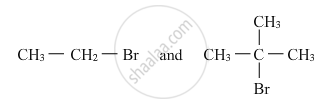
In the following pair of halogen compounds, which compound undergoes a faster SN1 reaction?
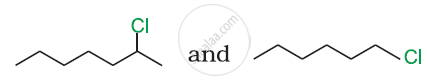
Which of the following pairs is/are correctly matched?
| Reaction | Product | |
| I | RX + AgCN | RNC |
| II | RX + KCN | RCN |
| III | RX + KNO2 | \[\begin{array}{cc} \phantom{.......}\ce{O}\\ \phantom{.....}/\\ \ce{R - N}\phantom{....}\\ \phantom{.....}\backslash\backslash\\ \phantom{.......}\ce{O} \end{array}\] |
| IV | RX + AgNO2 | \[\ce{R-O-N=O}\] |
Which of the following compounds is optically active?
Which of the following compound will undergo racemisation when reacts with aq. KOH?
(i)
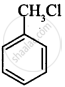
(ii)
CH3CH2CH2Cl
(iii)
\[\begin{array}{cc}
\ce{CH3}\phantom{..}\\
|\phantom{....}\\
\ce{CH3-CH-CH2Cl}
\end{array}\]
(iv)
\[\begin{array}{cc}
\phantom{..}\ce{H}\\
\phantom{..}|\\
\ce{CH3-C-Cl}\\
\phantom{..}|\\
\phantom{.....}\ce{C2H5}
\end{array}\]
Which of the following alkyl halides will undergo SN1 reaction most readily?
Which of the following compounds will show retention in configuration on nucleophile substitution by OH− ion?
Racemisation occurs in ______.
Discuss SN2 mechanism of methyl bromide using aqueous KOH.
Explain why Grignard reagents should be prepared under anhydrous conditions.
