Advertisements
Advertisements
Question
Why is it necessary to avoid even traces of moisture during the use of a Grignard reagent?
Solution
Grignard reagents are highly reactive and react with even traces of water to give corresponding hydrocarbons.
\[\ce{RMgX + H2O –>RH + Mg(OH)X}\]
APPEARS IN
RELATED QUESTIONS
Identify A, B, C, D, E, R and R1 in the following:

Which one of the following produces acyl halide by treatment with PCl5?
Which of the following statements are correct about the reaction intermediate?
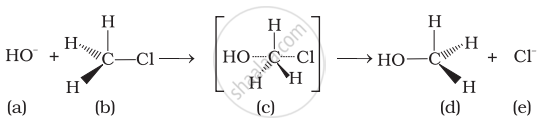
(i) Intermediate (c) is unstable because in this carbon is attached to 5 atoms.
(ii) Intermediate (c) is unstable because carbon atom is sp2 hybridised.
(iii) Intermediate (c) is stable because carbon atom is sp2 hybridised.
(iv) Intermediate (c) is less stable than the reactant (b).
Which of the statements about Grignard reagent is false?
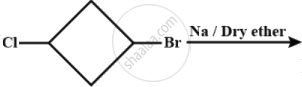 A product (1) of above reaction is:-
A product (1) of above reaction is:-
Explain why Grignard reagents should be prepared under anhydrous conditions?
Explain why Grignard reagents should be prepared under anhydrous conditions?
Explain why Grignard reagents should be prepared under anhydrous conditions?
Explain why Grignard reagents should be prepared under anhydrous conditions?
Explain why Grignard reagents should be prepared under anhydrous conditions?
