Advertisements
Advertisements
Question
Identify A, B, C, D, E, R and R1 in the following:

Solution
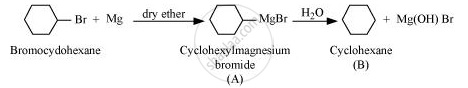
Since D of D2O gets attached to the carbon atom to which MgBr is attached, C is
\[\begin{array}{cc}
\ce{CH3CHCH3}\\
|\phantom{..}\\
\phantom{...}\ce{\underset{Isopropyl Magnesium bromide}{MgBr}}\
\end{array}\]
Therefore, the compound R−Br is
\[\begin{array}{cc}
\ce{CH3CHCH3}\\
|\phantom{..}\\
\ce{\underset{2-Bromopropane}{Br}}\
\end{array}\]
\[\begin{array}{cc}
\ce{CH3CHCH3 + Mg ->[dry ether] CH3CHCH3 ->[D2O] CH3CHCH3}\\
|\phantom{..........................}|\phantom{.................}|\phantom{..}\\
\ce{Br}\phantom{.......................}\ce{\underset{(C)}{MgBr}\phantom{.............}\ce{D}}\phantom{..}\\
\end{array}\]
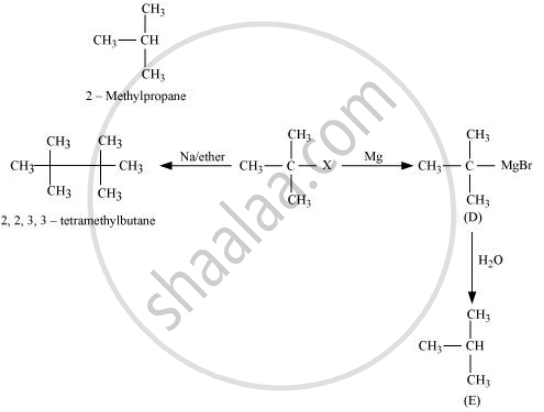
When an alkyl halide is treated with Na in the presence of ether, a hydrocarbon containing double the number of carbon atoms present in the original halide is obtained as a product. This is known as Wurtz reaction. Therefore, the halide, R1−X, is
\[\begin{array}{cc}
\phantom{.....}\ce{CH3}\\
\phantom{...}|\\
\ce{CH3 - C - X}\\
\phantom{...}|\\
\phantom{......}\ce{\underset{tert-Butylhalide}{CH3}}\
\end{array}\]
Therefore, compound D is
\[\begin{array}{cc}
\phantom{.}\ce{CH3}\\
|\phantom{..}\\
\ce{CH3 - C - MgBr}\\
|\phantom{..}\\
\phantom{.}\ce{\underset{tert-Butylmagnesiumbromide}{CH3}}\\
\end{array}\]
And, compound E is
APPEARS IN
RELATED QUESTIONS
How will you bring about the following conversion?
Bromomethane to propanone
Write the structure of the major organic product in the following reaction:
\[\ce{C6H5ONa + C2H5Cl ->}\]
How the following conversion can be carried out?
2-Chlorobutane to 3, 4-dimethylhexane
In the preparation of chlorobenzene from aniline, the most suitable reagent is:
Which of the following alkyl halides is used as a methylating agent?
Which of the following statements are correct about the reaction intermediate?
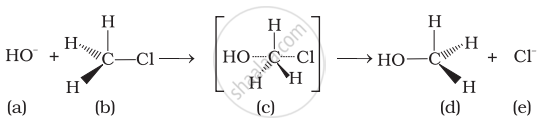
(i) Intermediate (c) is unstable because in this carbon is attached to 5 atoms.
(ii) Intermediate (c) is unstable because carbon atom is sp2 hybridised.
(iii) Intermediate (c) is stable because carbon atom is sp2 hybridised.
(iv) Intermediate (c) is less stable than the reactant (b).
Why is it necessary to avoid even traces of moisture during the use of a Grignard reagent?
Identify the following named reaction:
\[\ce{C2H5Br ->[Na/Dry ether] C2H5 - C2H5}\]
Reaction of Grignard reagent with aromatic aldehyde and subsequent aqueous treatment produces
Grignard reagent shows addition on
Explain why Grignard reagents should be prepared under anhydrous conditions?
Explain why Grignard reagents should be prepared under anhydrous conditions?
Explain why Grignard reagents should be prepared under anhydrous conditions?
Explain why Grignard reagents should be prepared under anhydrous conditions?
Explain why Grignard reagents should be prepared under anhydrous conditions?
Explain why Grignard reagents should be prepared under anhydrous conditions?
Explain why Grignard reagents should be prepared under anhydrous conditions?
