Advertisements
Advertisements
Question
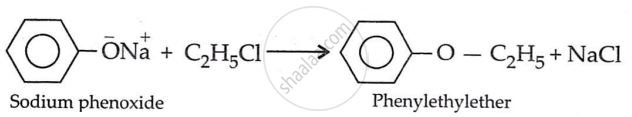
Write the structure of the major organic product in the following reaction:
\[\ce{C6H5ONa + C2H5Cl ->}\]
Solution 1
\[\ce{\underset{sodium phenoxide}{C6H5O^-Na} + \underset{ethyl chloride}{C2H5Cl} ->[Williamson Synthesis] \underset{phenetole}{C6H5 - O - C2H5} + NaCl}\]
Solution 2
Notes
Students can refer to the provided solutions based on their preferred marks.
APPEARS IN
RELATED QUESTIONS
How will you bring about the following conversion?
Bromomethane to propanone
What happens when methyl bromide is treated with sodium in the presence of dry ether?
How the following conversion can be carried out?
Chloroethane to butane
In the preparation of chlorobenzene from aniline, the most suitable reagent is:
Which of the following alkyl halides is used as a methylating agent?
What would be the reactant and reagent used to obtain 2, 4-dimethyl pentan-3-ol?
Draw other resonance structures related to the following structure and find out whether the functional group present in the molecule is ortho, para directing or meta directing.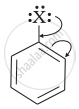
\[\ce{R - CH2 - CCl2 - R ->[Peagent] R - C ≅ C - R}\]. The reagent is:-
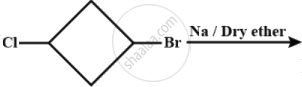 A product (1) of above reaction is:-
A product (1) of above reaction is:-
Grignard reagent shows addition on
The product formed in the first step of the reaction
\[\begin{array}{cc}
\ce{Br}\phantom{......}\\
|\phantom{.......}\\
\ce{CH3-CH2-CH-CH2-CH-CH3}\\
\phantom{...............}|\\
\phantom{................}\ce{Br}
\end{array}\]
with excess Mg/Et2O(Et = C2H5) is:
Explain why Grignard reagents should be prepared under anhydrous conditions?
Explain why Grignard reagents should be prepared under anhydrous conditions?
Explain why Grignard reagents should be prepared under anhydrous conditions?
Explain why Grignard reagents should be prepared under anhydrous conditions?
Explain why Grignard reagents should be prepared under anhydrous conditions?
