Advertisements
Advertisements
Question
In the following pair of halogen compounds, which compound undergoes a faster SN1 reaction?
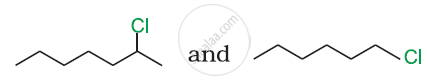
Solution
The activity of halogen compounds in SN1 reaction depends on the stability of the carbocation formed due to ionization. The order of stability is tertiary > secondary > primary. Hence, 2° alkyl chloride is more active than 1° alkyl chloride. Hence, 2° alkyl chloride is more active in SN1 reaction.
 reacts faster due to the greater stability of 2° carbocation than 1° carbocation.
reacts faster due to the greater stability of 2° carbocation than 1° carbocation.
APPEARS IN
RELATED QUESTIONS
Write the structure of an isomer of compound C4H9Br which is most reactive towards SN1 reaction
Which compound in the following pair will react faster in SN2 reaction with OH−?
(CH3)3CCl or CH3Cl
What happens when ethyl chloride is treated with aqueous KOH?
Identify 'A' in the following reaction -
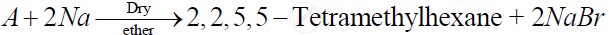
(a) 2- Bromo-2 methylbutane
(b) 1 -Bromo-2,2-dimethylpropane
(c) 1 - Bromo - 3 -methylbutane
(d) 1 - Bromo- 2 -methylpropane
Which would undergo SN2 reaction faster in the following pair and why ?
CH3 – CH2 – Br and CH3 – CH2 – I
What is the action of the following on ethyl bromide:
moist silver oxide
In a coordination entity of the type [PtCl2(en)2]2+ which isomer will show optical isomerism?
Arrange the following organic compounds in descending order of their reactivity towards SN1 reaction.
C6H5CH2Br, C6H5CH(C6H5)Br, C6H5CH(CH3)Br, C6H5C(CH3)(C6H5)Br
Which of the following pairs is/are correctly matched?
| Reaction | Product | |
| I | RX + AgCN | RNC |
| II | RX + KCN | RCN |
| III | RX + KNO2 | \[\begin{array}{cc} \phantom{.......}\ce{O}\\ \phantom{.....}/\\ \ce{R - N}\phantom{....}\\ \phantom{.....}\backslash\backslash\\ \phantom{.......}\ce{O} \end{array}\] |
| IV | RX + AgNO2 | \[\ce{R-O-N=O}\] |
Most reactive halide towards SN1 reaction is ____________.
In the SN1 reaction, racemization takes place. It is due to:
Which of the following is the correct order of decreasing SN2 reactivity?
An important chemical method to resolve a racemic mixture makes use of the formation of ______.
Among the following, the dissociation constant is highest for:
Which of the following alkyl halides will undergo SN1 reaction most readily?
Elimination reactions (especially β-elimination) are as common as the nucleophilic substitution reaction in case of alkyl halides. Specify the reagents used in both cases.
Aryl halides are extremely less reactive towards nucleophilic substitution. Predict and explain the order of reactivity of the following compounds towards nucleophilic substitution:
| (I) | 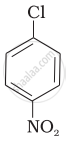 |
| (II) | 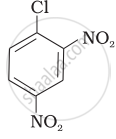 |
| (III) | 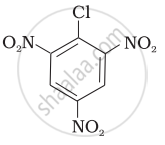 |
Why are aryl halides less reactive towards nucleophilic substitution reactions than alkyl halides? How can we enhance the reactivity of aryl halides?
Which one is the correct order of nucleophilic strength (pKa) of following nucleophiles?
Racemisation occurs in ______.
