Advertisements
Advertisements
Question
Why are aryl halides less reactive towards nucleophilic substitution reactions than alkyl halides? How can we enhance the reactivity of aryl halides?
Solution
Aryl halides are less reactive towards nucleophilic substitution reaction due to the following reasons.
(i) In haloarenes, the lone pair of electron on halogen are in resonance with benzene ring. So, C – Cl bond acquires partial double bond character which strengthen C – Cl bond and difficult to be substituted by nucleophile.
Therefore, they are less reactive towards nucleophilic substitution reaction.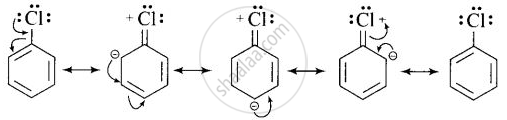
(ii) In haloarenes, the carbon atom attached to halogen is sp2 hybridised. The sp2 hybridised carbon is more electronegative than sp3 hybridised carbon. This sp2-hybridised carbon in haloarenes can hold the electron pair of \[\ce{C - X}\] bond more tightly and make this \[\ce{C - Cl}\] bond shorter than \[\ce{C Cl}\] bond of haloalkanes.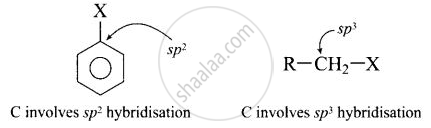
Since, it is difficult to break a shorter bond than a longer bond, therefore, halorenes are less reactive than haloalkanes.
(iii) In haloarenesm the phenyl cation is not stabilised by resonance therefore SN1 mechanism cannot be followed.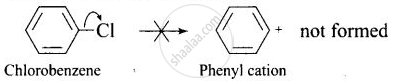
(iv) Because of the repulsion between the nucleophile and electron-rich arenas, aryl halides are less reactive than alkyl halides.
How to increase reactivity
The reactivity of aryl halides can be increased by the presence of an electron withdrawing group (-NO2) at ortho and para positions. No effect is observed by the presence of electron withdrawing group at weta-position Mechanism of the reaction is as depicted with OH ion:
The presence of NO2 groups at ortho and para positions withdraws electrons density from the benzene ring and therefore, facilitates the attack of the nucleophile on haloarenes. The carbanion, thus formed is stabilized through resonance as shown below:
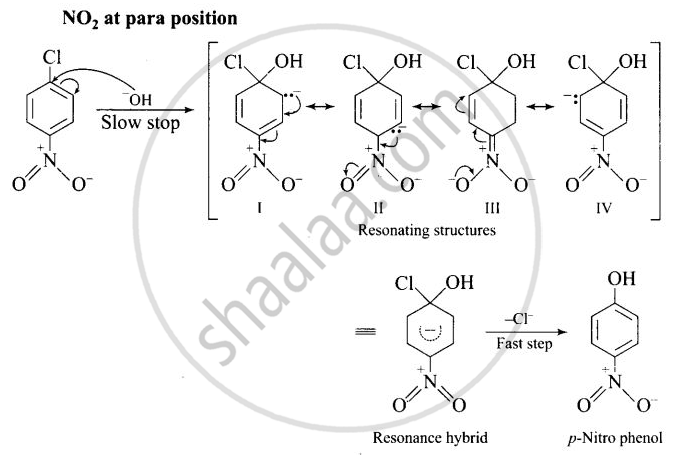
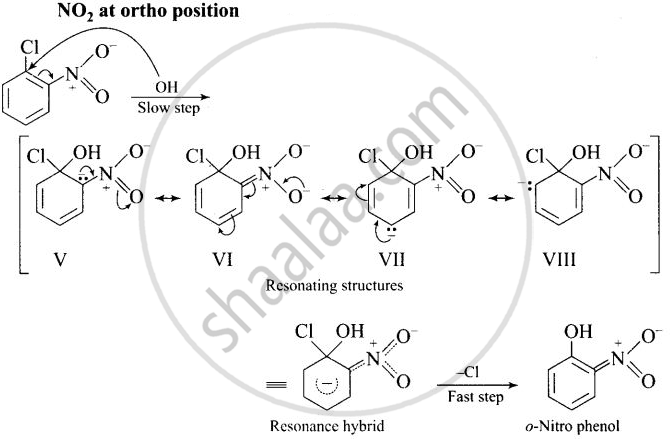
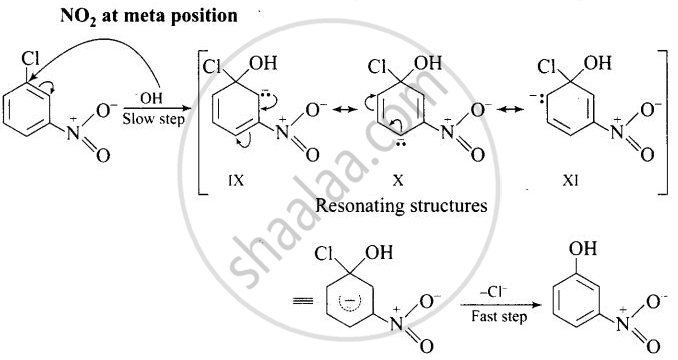
It is clear from above structure that in case of o- and p-chlorobenzenes, on the resonating structures (II in case of p-nitro chlorobenzene and V in case of o-chlorobenzene) bear a negative charge on the carbon atom bearing the \[\ce{NO2}\] group.
Therefore, these carbanions are stabilized by the -NO2 groups as well as π-electrons of the benzene ring. However, in case of m-nitrochlorobenzene, none of the resonating structures bear the negative charge on carbon atom bearing the -NO2 group. Therefore, the nitro group at meta position does not stabilize the negative charge but the carbanion is stabilized only by the p-electrons of the benzene ring. In other words, the carbanions formed from o-nitrochlorobenzene and p-nitrochlorobenzene are most stable than that formed from m-nitrochlorobenzcne.
Thus, the presence of electron withdrawing groups at o- and p-positions (but not at m-positions) w.r.t. halogen atom activates the aryl halides towards nucleophilic substitution reaction.
APPEARS IN
RELATED QUESTIONS
Out of 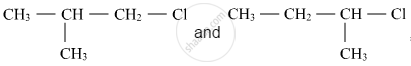 , which is more reactive towards SN1 reaction and why?
, which is more reactive towards SN1 reaction and why?
Which compound in the following pair will react faster in SN2 reaction with OH−?
CH3Br or CH3I
What is the action of the following on ethyl bromide?
moist silver oxide
In the reaction, \[\ce{R - X + NaOR' -> ROR’ + X}\] ( – ve ion). The main product formed is:
Isopropyl chloride undergoes hydrolysis by:
The process of separation of a racemic modification into d and l-enantiomers is called ____________.
Racemic compound has ____________.
CCl4 is insoluble in water because:-
Complete the reaction with the main product formed:

Identify the product in the following reaction: