Advertisements
Advertisements
Question
Aryl halides are extremely less reactive towards nucleophilic substitution. Predict and explain the order of reactivity of the following compounds towards nucleophilic substitution:
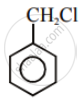
| (I) | 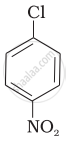 |
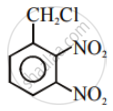
| (II) | 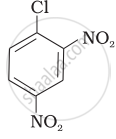 |
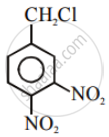
| (III) | 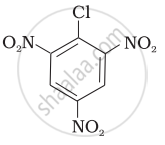 |
Solution
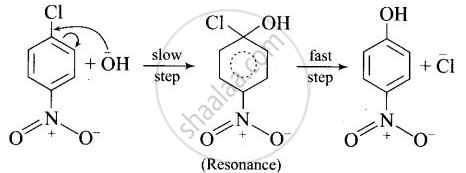
After the attachment of the nucleophile at the carbon carrying -Cl, the intermediate compound is stabilised due to resonance. Due to electron-withdrawing nature of-NO2, the nucleophile is easily attached to the benzene ring. Greater the number of -NO2 groups in the molecule, greater will be the ease with which the nucleophile will be attached. Hence, the order of reactivity is III > II > I.
APPEARS IN
RELATED QUESTIONS
Which alkyl halide from the following pair would you expect to react more rapidly by an SN2 mechanism? Explain your answer.
CH3CH2CH2CH2Br or \[\begin{array}{cc}
\ce{CH3CH2CHCH3}\\
\phantom{...}|\\
\phantom{....}\ce{Br}\
\end{array}\]
In the following pair of halogen compounds, which compound undergoes a faster SN1 reaction?
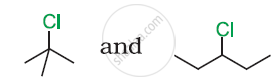
C–Cl bond length in chlorobenzene is shorter than C–Cl bond length in CH3–Cl.
What is the action of the following on ethyl bromide:
silver acetate
Which of the following is optically inactive?
Which of the following alkyl halides will undergo SN1 reaction most readily?
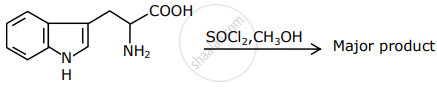
The major product formed in the following reaction is:
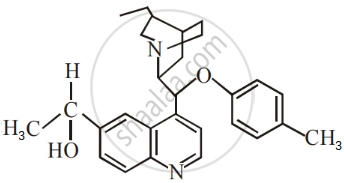
The number of chiral carbons present in the molecule given below is ______.
The decreasing order of reactivity of the following compounds towards nucleophilic substitution (SN2) is ______.
Which alkyl halide from the following pair would you expect to react more rapidly by an SN2 mechanism? Explain your answer.
\[\begin{array}{cc}\ce{CH3CHCH2CH2Br}\\|\phantom{.........}\\\ce{CH3}\phantom{......}\end{array}\] or \[\begin{array}{cc}\ce{CH3CH2CHCH2Br}\\\phantom{}|\\\phantom{...}\ce{CH3}\end{array}\]