Advertisements
Advertisements
Question
Which would undergo SN2 reaction faster in the following pair and why ?
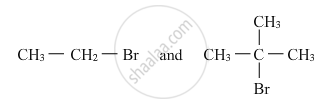
Solution
Primary alkyl halides prefer to undergo SN2 reactions than tertiary alkyl halides because of less steric hindrance experienced by the approaching nucleophile. Hence, out of the given pair (CH3−CH2−Br) would undergo SN2 reaction faster.
APPEARS IN
RELATED QUESTIONS
The treatment of alkyl chlorides with aqueous KOH leads to the formation of alcohols but in the presence of alcoholic KOH, alkenes are major products. Explain.
Which would undergo SN2 reaction faster in the following pair and why ?
CH3 – CH2 – Br and CH3 – CH2 – I
AgCN reacts with haloalkanes to form isocyanide. Haloalkanes react with KCN to form alkyl cyanides as the main product. Why?
Isopropyl chloride undergoes hydrolysis by:
Which of the following is a chiral compound?
The reaction of C6H5–CH=CH–CH3 with HBr produces:
A primary alkyl halide would prefer to undergo ______.
Which of the compounds will react faster in SN1 reaction with the –OH ion?
\[\ce{CH3-CH2-Cl}\] or \[\ce{C6H5-CH2-Cl}\]
Which of the following compounds will show retention in configuration on nucleophile substitution by OH− ion?
Retention of configuration is observed in ______.
