Advertisements
Advertisements
Question
The treatment of alkyl chlorides with aqueous KOH leads to the formation of alcohols but in the presence of alcoholic KOH, alkenes are major products. Explain.
Solution 1
In an aqueous solution, KOH almost completely ionizes to give OH– ion, a strong nucleophile and reacts with alkyl halides to form alcohols. In an aqueous solution, OH– ions are highly hydrated. This reduces the basic character of OH– ions, due to which they fail to separate hydrogen atoms from the β-carbon of alkyl halide and cannot form an alkene.
On the other hand, alcoholic solution of KOH contains alkoxide (RO–) ions which, being a stronger base than OH–, easily remove HCl molecule from alkyl chloride to form alkene.
Solution 2
Simple nucleophilic substitution occurs when alkyl chlorides react with aqueous KOH to form alcohols.
\[\ce{CH3 - CH2 - Cl + KOH ->[H2O]CH3 - CH2 - OH + KCl}\]
When aqueous KOH is substituted with alcoholic KOH, HCI is eliminated from an alkyl halide, resulting in the formation of alkenes instead of alcohols.
\[\ce{CH3 - CH2Cl + KOH->[EtOH] CH2 = CH2}\]
This can be explained by the size of the nucleophile in both reactions. In an aqueous medium, the \[\ce{N\overset{Θ}{u}}\] is \[\ce{\overset{Θ}{O}H}\] which is small, whereas in an alcoholic medium, the \[\ce{N\overset{Θ}{u}}\] is \[\ce{C2H^Θ5}\] is bulky.
The bulky \[\ce{N\overset{Θ}{u}}\] always find it easier to abstract a proton rather than attack a tetravalent carbon and form a substitution product.
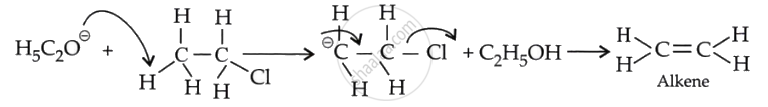
If C2H5OΘ attacks a carbon-carrying halogen, steric repulsions can delay the attack and prevent substitution.
Notes
Students can refer to the provided solutions based on their preferred marks.
APPEARS IN
RELATED QUESTIONS
Write the mechanism of the following reaction:
\[\ce{{n}BuBr + KCN ->[EtOH-H2O] {n}BuCN}\]
In the reaction, \[\ce{R - X + NaOR' -> ROR’ + X}\] ( – ve ion). The main product formed is:
Which of the following is optically inactive?
Most reactive halide towards SN1 reaction is ____________.
In the SN1 reaction, racemization takes place. It is due to:
Isopropyl chloride undergoes hydrolysis by:
Racemic compound has ____________.
An organic molecule necessarily shows optical activity if it ____________.
The correct order of increasing the reactivity of C–X bond towards nucleophile in following compounds.
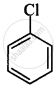
(I)
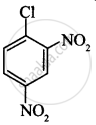
(II)
(CH3)3CCl
(III)
(CH3)2CHCl
(IV)
Which of the following statements are correct about this reaction?
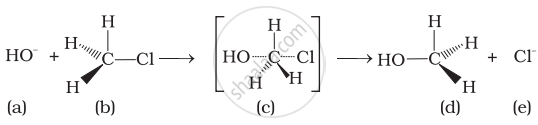
(i) The given reaction follows SN2 mechanism.
(ii) (b) and (d) have opposite configuration.
(iii) (b) and (d) have same configuration.
(iv) The given reaction follows SN1 mechanism.
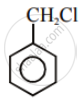
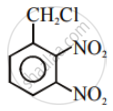
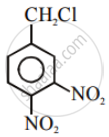
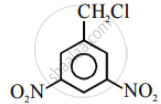
Write the structures and names of the compounds formed when compound ‘A’ with molecular formula, \[\ce{C7H8}\] is treated with \[\ce{Cl2}\] in the presence of \[\ce{FeCl3}\].
Chlorination of alkanes is an example of
Which one is the correct order of nucleophilic strength (pKa) of following nucleophiles?
When CH3CH2CHCl2 is treated NaNH2 product formed is:-
The decreasing order of reactivity of the following compounds towards nucleophilic substitution (SN2) is ______.
The following questions are case-based questions. Read the passage carefully and answer the questions that follow:
|
Nucleophilic Substitution: Influences of solvent polarity: The reaction rate (SN2) of 2-bromopropane and NaOH in ethanol containing 40% water is twice slower than in absolute ethanol. Hence the level of solvent polarity has an influence on both SN1 and SN2 reactions but with different results. Generally speaking, a weak polar solvent is favourable for SN2 reaction, while a strong polar solvent is favourable for SN1. Generally speaking, the substitution reaction of tertiary haloalkane is based on SN1 mechanism in solvents with a strong polarity (for example ethanol containing water). |
Answer the following questions:
(a) Why racemisation occurs in SN1? (1)
(b) Why is ethanol less polar than water? (1)
(c) Which one of, the following in each pair is more reactive towards SN2 reaction? (2)
(i) CH3 – CH2 – I or CH3CH2 – Cl
(ii)
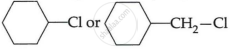
OR
(c) Arrange the following in the increasing order of their reactivity towards SN1 reactions: (2)
(i) 2-Bromo-2-methylbutane, 1-Bromo-pentane, 2-Bromo-pentane
(ii) 1-Bromo-3-methylbutane, 2-Bromo-2-methylbutane, 2-Bromo-3- methylbutane
Discuss SN2 mechanism of methyl bromide using aqueous KOH.
Explain why Grignard reagents should be prepared under anhydrous conditions.
Which alkyl halide from the following pair would you expect to react more rapidly by an SN2 mechanism? Explain your answer.
\[\begin{array}{cc}\ce{CH3CH2CHCH3}\\\phantom{...}|\\\phantom{....}\ce{Br}\end{array}\] or \[\begin{array}{cc}\phantom{.....}\ce{CH3}\\\phantom{..}|\\\ce{H3C - C - Br}\\\phantom{..}|\\\phantom{....}\ce{CH3}\end{array}\]