Advertisements
Advertisements
Question
Which alkyl halide from the following pair would you expect to react more rapidly by an SN2 mechanism? Explain your answer.
\[\begin{array}{cc}\ce{CH3CH2CHCH3}\\\phantom{...}|\\\phantom{....}\ce{Br}\end{array}\] or \[\begin{array}{cc}\phantom{.....}\ce{CH3}\\\phantom{..}|\\\ce{H3C - C - Br}\\\phantom{..}|\\\phantom{....}\ce{CH3}\end{array}\]
Solution
The SN2 process involves a transition state with both an incoming nucleophile and a leaving group surrounding the carbon atom. Five atoms are simultaneously bonded together. A transition state requires minimal steric hindrance. Hence, 1° alkyl halides are the most reactive to SN2, followed by 2° and 3°.
1° RX > 2° RX > 3° RX
Based on the above order, \[\begin{array}{cc}
\ce{CH3CH2CHCH3}\\
\phantom{...}|\\
\phantom{....}\ce{Br}\
\end{array}\] is more reactive.
RELATED QUESTIONS
Write the major products(s) in the following:
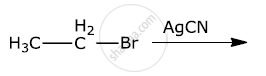
What is the action of the following on ethyl bromide?
moist silver oxide
In the SN1 reaction, racemization takes place. It is due to:
The order of reactivities of the following alkyl halides for an SN2 reaction is:
Which of the following alkyl halides will undergo SN1 reaction most readily?
Complete the following analogy:
Same molecular formula but different structures: A : : Non superimposable mirror images: B
Which of the following statements are correct about the kinetics of this reaction?
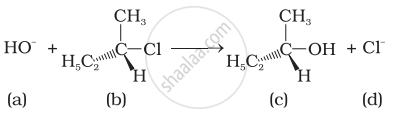
(i) The rate of reaction depends on the concentration of only (b).
(ii) The rate of reaction depends on concentration of both (a) and (b).
(iii) Molecularity of reaction is one.
(iv) Molecularity of reaction is two.
Ethylene chloride and ethylidene chloride are isomers. Identify the correct statements.
(i) Both the compounds form same product on treatment with alcoholic KOH.
(ii) Both the compounds form same product on treatment with aq.NaOH.
(iii) Both the compounds form same product on reduction.
(iv) Both the compounds are optically active.
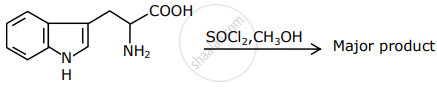
The major product formed in the following reaction is:
Explain why Grignard reagents should be prepared under anhydrous conditions.
