Advertisements
Advertisements
प्रश्न
Which alkyl halide from the following pair would you expect to react more rapidly by an SN2 mechanism? Explain your answer.
\[\begin{array}{cc}\ce{CH3CH2CHCH3}\\\phantom{...}|\\\phantom{....}\ce{Br}\end{array}\] or \[\begin{array}{cc}\phantom{.....}\ce{CH3}\\\phantom{..}|\\\ce{H3C - C - Br}\\\phantom{..}|\\\phantom{....}\ce{CH3}\end{array}\]
उत्तर
The SN2 process involves a transition state with both an incoming nucleophile and a leaving group surrounding the carbon atom. Five atoms are simultaneously bonded together. A transition state requires minimal steric hindrance. Hence, 1° alkyl halides are the most reactive to SN2, followed by 2° and 3°.
1° RX > 2° RX > 3° RX
Based on the above order, \[\begin{array}{cc}
\ce{CH3CH2CHCH3}\\
\phantom{...}|\\
\phantom{....}\ce{Br}\
\end{array}\] is more reactive.
संबंधित प्रश्न
In the following pair of halogen compounds, which compound undergoes a faster SN1 reaction?
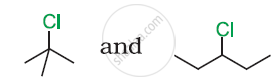
Which would undergo SN2 reaction faster in the following pair and why ?
CH3 – CH2 – Br and CH3 – CH2 – I
AgCN reacts with haloalkanes to form isocyanide. Haloalkanes react with KCN to form alkyl cyanides as the main product. Why?
Which one is most reactive towards SN1 reaction?
The process of separation of a racemic modification into d and l-enantiomers is called ____________.
An organic molecule necessarily shows optical activity if it ____________.
Give reason for the following:
The product formed during SN1 reaction is a racemic mixture.
Optical activity of an enantiomeric mixture is +12.6° and the specific rotation of (+) isomer is +30°. The optical purity is ______ %.
Arrange the following compounds in increasing order of reactivity towards SN2 reaction.
2-Bromopentane, 1-Bromopentane, 2-Bromo-2-methylbutane
Identify the product in the following reaction: