Advertisements
Advertisements
Question
Write the major products(s) in the following:
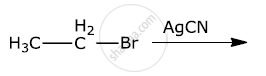
Solution
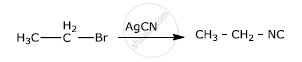
APPEARS IN
RELATED QUESTIONS
Discuss the mechanism of alkaline hydrolysis of bromomethane.
Which would undergo SN1 reaction faster in the following pair and why?
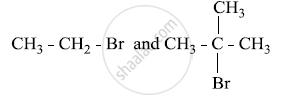
The treatment of alkyl chlorides with aqueous KOH leads to the formation of alcohols but in the presence of alcoholic KOH, alkenes are major products. Explain.
SN2 mechanism proceeds through intervention of ____________.
The process of separation of a racemic modification into d and l-enantiomers is called ____________.
Compound ‘A’ with molecular formula \[\ce{C4H9Br}\] is treated with aq. \[\ce{KOH}\] solution. The rate of this reaction depends upon the concentration of the compound ‘A’ only. When another optically active isomer ‘B’ of this compound was treated with aq. \[\ce{KOH}\] solution, the rate of reaction was found to be dependent on concentration of compound and \[\ce{KOH}\] both.
(i) Write down the structural formula of both compounds ‘A’ and ‘B’.
(ii) Out of these two compounds, which one will be converted to the product with inverted configuration.
Which one is the correct order of nucleophilic strength (pKa) of following nucleophiles?
Which of the following compounds will show retention in configuration on nucleophile substitution by OH− ion?
Acetic anhydride from acetic acid
Discuss the mechanism of alkaline hydrolysis of methyl bromide.
