Advertisements
Advertisements
प्रश्न
Which would undergo SN2 reaction faster in the following pair and why ?
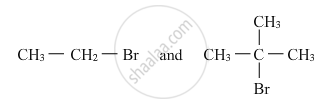
उत्तर
Primary alkyl halides prefer to undergo SN2 reactions than tertiary alkyl halides because of less steric hindrance experienced by the approaching nucleophile. Hence, out of the given pair (CH3−CH2−Br) would undergo SN2 reaction faster.
APPEARS IN
संबंधित प्रश्न
Give reasons for the following:
(CH3)3C–O–CH3 on reaction with HI gives (CH3)3C–I and CH3–OH as the main products and not (CH3)3C–OH and CH3–I.
Arrange the compounds of the following set in order of reactivity towards SN2 displacement:
1-Bromobutane, 1-Bromo-2, 2-dimethylpropane, 1-Bromo-2-methylbutane, 1-Bromo-3-methylbutane
C–Cl bond length in chlorobenzene is shorter than C–Cl bond length in CH3–Cl.
Which would undergo SN2 reaction faster in the following pair and why ?
CH3 – CH2 – Br and CH3 – CH2 – I
What is the action of the following on ethyl bromide:
silver acetate
Racemic compound has ____________.
Which of the compounds will react faster in SN1 reaction with the –OH ion?
\[\ce{CH3-CH2-Cl}\] or \[\ce{C6H5-CH2-Cl}\]
Write the structures and names of the compounds formed when compound ‘A’ with molecular formula, \[\ce{C7H8}\] is treated with \[\ce{Cl2}\] in the presence of \[\ce{FeCl3}\].
How do polar solvents help in the first step in SN1 mechanism?
CCl4 is insoluble in water because:-
