Advertisements
Advertisements
Question
Arrange the compounds of the following set in order of reactivity towards SN2 displacement:
1-Bromo-3-methylbutane, 2-Bromo-2-methylbutane, 2-Bromo-3-methylbutane
Solution
The SN2 reaction involves the formation of a transition state with the carbon atom surrounded by 5 additional atoms (groups). A transition state requires minimum steric interactions. The most suitable substrates for SN2 reactions are 1° alkyl halides, followed by 2° and 3° alkyl halides. The order of reactivity towards SN2 is 1° > 2° > 3°> aryl halide. Based on this, the order will be
\[\begin{array}{cc}
\ce{CH3}\phantom{...............................}\ce{CH3}\phantom{.................}\ce{CH3}\phantom{.....}\\
\phantom{.....}|\phantom{...................................}|\phantom{....................}|\phantom{............}\\
\ce{\underset{1-Bromo-3-methylbutane}{H3C - CH - CH2 - CH2Br} > \ce{CH3 - CH - CH - CH3} > \ce{CH3 - C - CH2 - CH3}}\\
\phantom{.......................}|\phantom{..........................}|\\
\phantom{.........................}\ce{\underset{2-Bromo-3-methylbutane}{Br}\phantom{.........}\ce{\underset{2-Bromo-2-methylbutane}{Br}}}\
\end{array}\]
APPEARS IN
RELATED QUESTIONS
Given reasons: C–Cl bond length in chlorobenzene is shorter than C–Cl bond length in CH3–Cl.
Write the isomers of the compound having the formula C4H9Br.
The stability order for carbocation is _______.
(A) 2° > 3° > 1°
(B) 3° > 2° > 1°
(C) 3° > 1° > 2°
(D) 1° > 3° > 2°
Which compound in the following pair reacts faster in SN2 reaction with OH–?
- CH3Br or CH3
- CH3Cl, (CH3)3CCl
Which of the following is optically inactive?
Halogenation of alkanes is ____________.
Which among MeX, RCH2X, R2CHX and R3CX is most reactive towards SN2 reaction?
Tertiary alkyl halides are practically inert to substitution by SN2 mechanism because of ____________.
Which of the following is an optically active compound?
Which of the following compounds is optically active?
Which of the following is a chiral compound?
Identify X and Y in the following sequence:
\[\ce{C2H5Br ->[X] Product ->[Y] C3H7NH2}\]
Which of the following compound will undergo racemisation when reacts with aq. KOH?
(i)
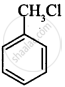
(ii)
CH3CH2CH2Cl
(iii)
\[\begin{array}{cc}
\ce{CH3}\phantom{..}\\
|\phantom{....}\\
\ce{CH3-CH-CH2Cl}
\end{array}\]
(iv)
\[\begin{array}{cc}
\phantom{..}\ce{H}\\
\phantom{..}|\\
\ce{CH3-C-Cl}\\
\phantom{..}|\\
\phantom{.....}\ce{C2H5}
\end{array}\]
Chlorination of alkanes is an example of
In which reaction mechanism carbocation is formed?
In SN1 reactions, the correct order of reactivity for the following compounds:
CH3Cl, CH3CH2Cl, (CH3)2CHCl and (CH3)3CCl is ______.
Arrange the following compounds in increasing order of reactivity towards SN2 reaction.
2-Bromopentane, 1-Bromopentane, 2-Bromo-2-methylbutane
Inversion of configuration occurs in ______.
Discuss the mechanism of alkaline hydrolysis of methyl bromide.
Explain why Grignard reagents should be prepared under anhydrous conditions.
