Advertisements
Advertisements
Question
Write the isomers of the compound having the formula C4H9Br.
Solution
C4H9Br is a saturated compound because its parent hydrocarbon is C4H10. Its isomers are as follows –
(i) \[\ce{\underset{1-Bromobutane}{CH3 - CH2 - CH2 - CH2 - Br}}\]
(ii) \[\begin{array}{cc}
\phantom{.......}\ce{Br}\\
\phantom{.....}|\\
\ce{\underset{2-Bromobutane}{CH3-CH2-CH-CH3}}
\end{array}\]
(iii) \[\begin{array}{cc}
\ce{CH3}\phantom{....}\\
|\phantom{.......}\\
\ce{\underset{1-Bromo-2-Methylpropane}{CH3-CH-CH2Br}}
\end{array}\]
(iv) \[\begin{array}{cc}
\ce{CH3}\\
|\phantom{...}\\
\ce{CH3 - C - Br}\phantom{.....}\\
|\phantom{...}\\
\phantom{.}\ce{\underset{2-Bromo-2-Methylpropane}{CH3}}
\end{array}\]
APPEARS IN
RELATED QUESTIONS
How do you convert the following:
Ethanol to propanenitrile
Give reasons for the following:
(CH3)3C–O–CH3 on reaction with HI gives (CH3)3C–I and CH3–OH as the main products and not (CH3)3C–OH and CH3–I.
Out of 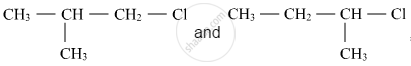 , which is more reactive towards SN1 reaction and why?
, which is more reactive towards SN1 reaction and why?
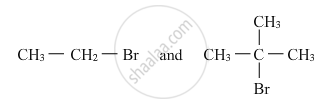
Which would undergo SN2 reaction faster in the following pair and why ?
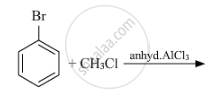
Write the structure of the major product in each of the following reaction :
Write the structure of the major organic product in the following reaction:
\[\ce{CH3CH2Br + KCN ->[aq.ethanol]}\]
Write the mechanism of the following reaction:
\[\ce{{n}BuBr + KCN ->[EtOH-H2O] {n}BuCN}\]
What happens when methyl chloride is treated with KCN?
Arrange the following organic compounds in descending order of their reactivity towards SN1 reaction.
C6H5CH2Br, C6H5CH(C6H5)Br, C6H5CH(CH3)Br, C6H5C(CH3)(C6H5)Br
The increasing order of reactivity towards SN1 mechanism is:
(I) \[\begin{array}{cc}
\ce{CH3-CH-CH2-CH3}\\
|\phantom{........}\\
\ce{CH3}\phantom{.....}
\end{array}\]
(II) CH3CH2CH2Cl
(III) P–CH3O–C6H4–CH2Cl
Which of the compounds will react faster in SN1 reaction with the –OH ion?
\[\ce{CH3-CH2-Cl}\] or \[\ce{C6H5-CH2-Cl}\]
Write the structures and names of the compounds formed when compound ‘A’ with molecular formula, \[\ce{C7H8}\] is treated with \[\ce{Cl2}\] in the presence of \[\ce{FeCl3}\].
Chlorination of alkanes is an example of
Which one of the following compounds is more reactive towards SN1 reaction?
The number of chiral carbons present in the molecule given below is ______.
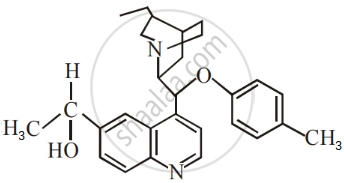
Retention of configuration is observed in ______.
Complete the reaction with the main product formed:

Which alkyl halide from the following pair would you expect to react more rapidly by an SN2 mechanism? Explain your answer.
\[\begin{array}{cc}\ce{CH3CH2CHCH3}\\\phantom{...}|\\\phantom{....}\ce{Br}\end{array}\] or \[\begin{array}{cc}\phantom{.....}\ce{CH3}\\\phantom{..}|\\\ce{H3C - C - Br}\\\phantom{..}|\\\phantom{....}\ce{CH3}\end{array}\]
