Advertisements
Advertisements
Question
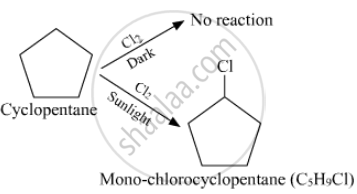
A hydrocarbon C5H10 does not react with chlorine in dark but gives a single monochloro compound C5H9Cl in bright sunlight. Identify the hydrocarbon.
Solution
A hydrocarbon with the molecular formula C5H10 belongs to the group with a general molecular formula CnH2n. Therefore, it may either be an alkene or a cycloalkane.
Since hydrocarbon does not react with chlorine in the dark, it cannot be an alkene. Thus, it should be a cycloalkane.
Further, the hydrocarbon gives a single monochloro compound, C5H9Cl by reacting with chlorine in bright sunlight. Since a single monochloro compound is formed, the hydrocarbon must contain H−atoms that are all equivalent. Also, as all H−atoms of a cycloalkane are equivalent, the hydrocarbon must be a cycloalkane. Hence, the said compound is cyclopentane. The reactions involved are:
APPEARS IN
RELATED QUESTIONS
Why is sulphuric acid not used during the reaction of alcohols with KI?
Draw the structure of major monohalo products in the following reaction:
\[\ce{CH3CH2Br + NaI ->}\]
Write the equation for the preparation of 1-iodobutane from 1-butanol.
How will you bring about the following conversion?
Ethanol to but-1-yne
How will you bring about the following conversion?
Propene to 1-nitropropane
How will you bring about the following conversion?
Propene to propyne
How will you bring about the following conversion?
Ethanol to ethyl fluoride
Write the structure of the major organic product in the following reaction:
\[\ce{CH3CH2CH2Cl + NaI ->[acetone][heat]}\]
Write the structure of the major organic product in the following reaction:
\[\ce{CH3CH2CH = CH2 + HBr->[peroxide]}\]
Write the structure of the major organic product in the following reaction:
\[\ce{CH3CH = C(CH3)2 + HBr ->}\]
Primary alkyl halide C4H9Br (a) reacted with alcoholic KOH to give compound (b). Compound (b) is reacted with HBr to give (c) which is an isomer of (a). When (a) is reacted with sodium metal it gives compound (d), C8H18 which is different from the compound formed when n-butyl bromide is reacted with sodium. Give the structural formula of (a) and write the equations for all the reactions.
How the following conversion can be carried out?
Benzyl alcohol to 2-phenylethanoic acid
How the following conversion can be carried out?
2-Methyl-1-propene to 2-chloro-2-methylpropane
How the following conversion can be carried out?
tert-Butyl bromide to isobutyl bromide
Among the isomeric alkanes of molecular formula C5H12, identify the one that on photochemical chlorination yields four isomeric monochlorides.
