Advertisements
Advertisements
Question
Which one of the following has the highest dipole moment?
Options
CH2Cl2
CHCl3
CCl4
MCQ
Solution
CH2Cl2
Explanation:
- In CH2Cl2, the resultant of two C-Cl dipole moments is strengthened by two C-H dipoles, resulting in the highest dipole moment (1.62 D).
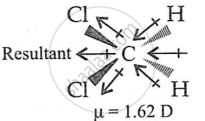
- In CHCl3, the resultant of two C-Cl dipoles is opposite the resultant of C-H and C-Cl bonds. Because the latter is expected to be smaller than the former, CHCl3 has a dipole moment (1.03 D).

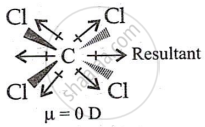
- CCl4 has a perfectly symmetrical structure with a net dipole moment of 4C-Cl = 0.
shaalaa.com
Is there an error in this question or solution?
Chapter 10: Haloalkanes and Haloarenes - Exercises [Page 311]
APPEARS IN
RELATED QUESTIONS
which of the following carbocations is least stable?
(A) 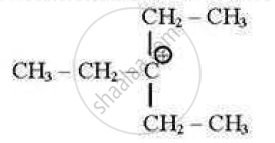
(B)![]()
(C)![]()
(D)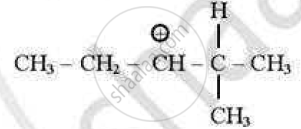
C – X bond is strongest in ____________.
Which of the following will have the maximum dipole moment?
Write a test to detect the presence of double bond in a molecule.
Discuss the nature of C – X bond in the haloarenes.
Assertion: Nitration of chlorobenzene leads to the formation of m-nitrochlorobenzene.
Reason: –NO2 group is a m-directing group.
Out of the Chloromethane and Fluoromethane, which one is has higher dipole moment and why?
Why haloarenes are Less reactive than halo alkanes?
