Advertisements
Advertisements
Questions
How do you convert the following:
Ethanol to propanenitrile
How the following conversion can be carried out?
Ethanol to propanenitrile
Solution 1
\[\ce{\underset{Ethanol}{CH3 - CH2 - OH} ->[red P/Br2] \underset{Bromoethane}{CH3 - CH2 - Br} ->[KCN, aq.ethanol]\underset{Propanenitrile}{CH3 - CH2 - CN}}\]
Solution 2
\[\ce{\underset{Ethanol}{CH3CH2OH} ->[P/I2, \Delta] \underset{Iodoethane}{CH3CH2I} ->[KCN/EtOH-H2O][nucleophilic substitution] \underset{Propanenitrile}{CH3CH2CN}}\]
Notes
Students can refer to the provided solutions based on their preferred marks.
APPEARS IN
RELATED QUESTIONS
Write the structure of an isomer of compound C4H9Br which is most reactive towards SN1 reaction
Write the structure of the major product in each of the following reaction :
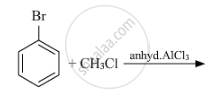
Write the structures of A, B and C in the following:

What is the action of the following on ethyl bromide:
moist silver oxide
In the reaction, \[\ce{R - X + NaOR' -> ROR’ + X}\] ( – ve ion). The main product formed is:
Which compound in the following pair reacts faster in SN2 reaction with OH–?
- CH3Br or CH3
- CH3Cl, (CH3)3CCl
Which of the following pairs is/are correctly matched?
| Reaction | Product | |
| I | RX + AgCN | RNC |
| II | RX + KCN | RCN |
| III | RX + KNO2 | \[\begin{array}{cc} \phantom{.......}\ce{O}\\ \phantom{.....}/\\ \ce{R - N}\phantom{....}\\ \phantom{.....}\backslash\backslash\\ \phantom{.......}\ce{O} \end{array}\] |
| IV | RX + AgNO2 | \[\ce{R-O-N=O}\] |
Which of the following is a primary halide?
Optically active isomers but not mirror images are called ____________.
Which of the following is a chiral compound?
Which of the following undergoes nucleophilic substitution exclusively by SN1 mechanism?
The reaction of C6H5–CH=CH–CH3 with HBr produces:
Which reagent will you use for the following reaction?
\[\ce{CH3CH2CH2CH3 -> CH3CH2CH2CH2Cl + CH3CH2CHClCH3}\]
Write the structures and names of the compounds formed when compound ‘A’ with molecular formula, \[\ce{C7H8}\] is treated with \[\ce{Cl2}\] in the presence of \[\ce{FeCl3}\].
Elimination reactions (especially β-elimination) are as common as the nucleophilic substitution reaction in case of alkyl halides. Specify the reagents used in both cases.
Which one is the correct order of nucleophilic strength (pKa) of following nucleophiles?
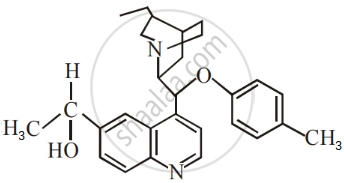
The number of chiral carbons present in the molecule given below is ______.
In SN1 reactions, the correct order of reactivity for the following compounds:
CH3Cl, CH3CH2Cl, (CH3)2CHCl and (CH3)3CCl is ______.
Racemisation occurs in ______.
Inversion of configuration occurs in ______.
